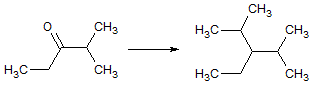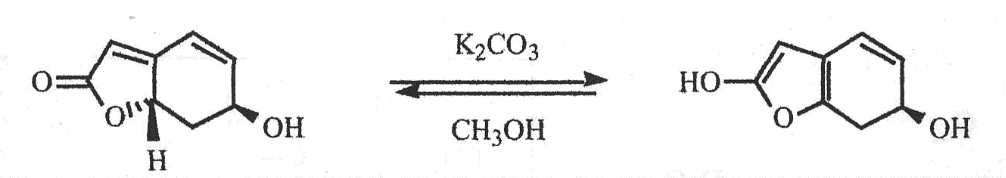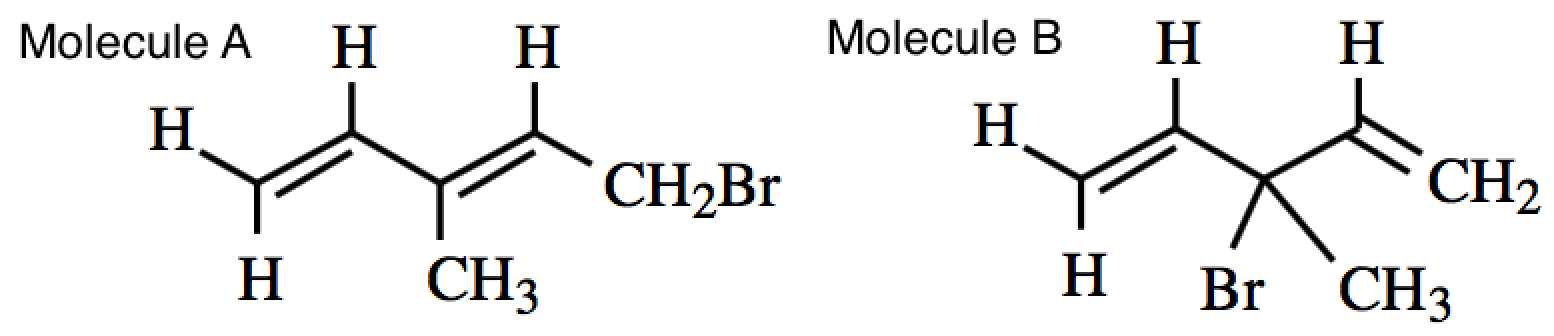|
| 1 |
Go |
Q:
|
Increased steric hindrance enhances the rate of SN1 reactions versus SN2 reactions because |
|
A
|
it is more difficult for the nucleophile to attack a sterically-hindered molecule. |
B
|
steric hindrance increases reactivity which makes SN1 reactions more likely. |
C
|
steric hindrance protects the nucleophile from reacting with the solvent. |
D
|
nucleophilic attack of sterically-hindered molecules is easier than non-sterically-hindered molecules. |
|
|
|
Tags:
Organic Chemistry Reactions | Molecular Structure | |
|
| 3 |
Go |
Q:
|
Hydroformylation involves the addition of a formaldehyde (formyl groupe) onto alkenes. For a hydroformylation process with propene that follows Markovnikov addition using a carbocation intermediate, which product will be favored? |
|
A
|
butanal |
B
|
2-methylpropanal |
C
|
2-methylbutanal |
D
|
propanal |
|
|
|
Tags:
Organic Chemistry Reactions | Molecular Structure | |
|
| 4 |
Go |
Q:
|
The reaction below depicts a 1,2-hydride shift which is a reaction that heavily favors the products.

The reason for strongly favoring the products is:
|
|
A
|
the primary cabocation is more charged than the secondary carbocation and pulls the hydrogen atom toward itself |
B
|
the hydride shift favors a secondary carbocation rather than a primary carbocation |
C
|
methyl groups are more electronegative and pull positive charges toward themselves |
D
|
the secondary carbocation is less stericaly hindered than the primary carbocation |
|
|
|
Tags:
Organic Chemistry Reactions | Molecular Structure | |
|
| 5 |
Go |
Q:
|
Upon oxidation with excess reagents, which of the following choices best describes the resultant molecule in terms of carboxylic acid and ketone sites present?
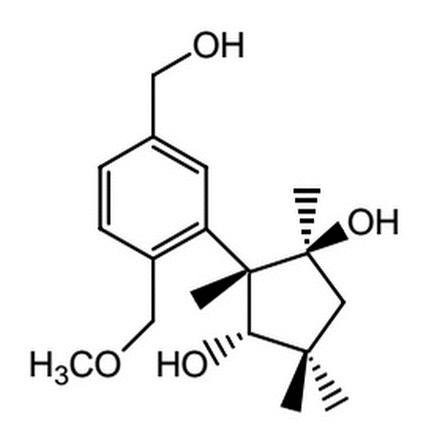 |
|
A
|
One carboxylic acid, two ketones |
B
|
Three ketones |
C
|
Two carboxylic acids |
D
|
One carboxylic acid, one ketone |
|
|
|
Tags:
Carboxylic Acids | Organic Chemistry Reactions | |
|
| 7 |
Go |
Q:
|
In each molecule below, a carbocation is formed when the alcohol group is removed. Which of the following best describes the stabilities of the carbocations?
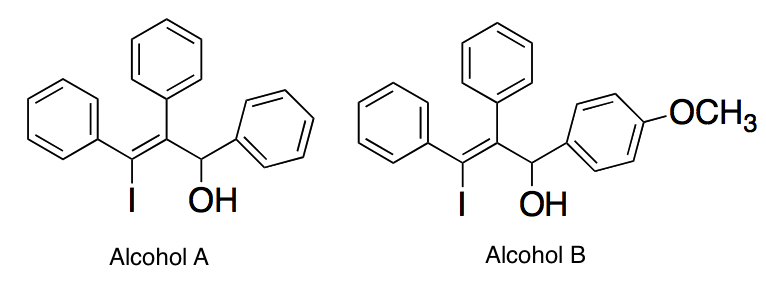
|
|
A
|
The carbocation of Alcohol A is more stable than the carbocation of Alcohol B since it lacks a methoxy group, which would destabilize the carbocation
|
B
|
The carbocation of Alcohol A is less stable than the carbocation of Alcohol B since it lacks a methoxy group, which would destabilize the carbocation
|
C
|
The carbocation of Alcohol B is more stable than the carbocation of Alcohol A since it has a methoxy group, which would stabilize the carbocation
|
D
|
The carbocation of Alcohol B is less stable than the carbocation of Alcohol A since it has a methoxy group, which would stabilize the carbocation
|
|
|
|
Tags:
Molecular Structure | Organic Chemistry Reactions | |
|
| 9 |
Go |
Q:
|
In the following reaction, the fastest reaction rate occurs in pure water, as opposed to occurring in a solvent like ethanol. Which of the following best describes why this scenario takes place?
 |
|
A
|
The aromatic rings in both reactants are hydrophobic and will be in close proximity to each other, which increases the chance of a reaction taking place
|
B
|
The aromatic rings in both reactants are hydrophobic and will be in close proximity to each other, which decreases the chance of a reaction taking place
|
C
|
The aromatic rings in both reactants are hydrophilic and will be in close proximity to each other, which increases the chance of a reaction taking place
|
D
|
The aromatic rings in both reactants are hydrophilic and will be in close proximity to each other, which decreases the chance of a reaction taking place
|
|
|
|
Tags:
Carboxylic Acids | Molecular Structure | Organic Chemistry Reactions | |
|
| 10 |
Go |
Q:
|
An allylic substitution reaction results in two regioisomeric monobromides, as shown below. Which of the following statements about their relative stabilities is correct?
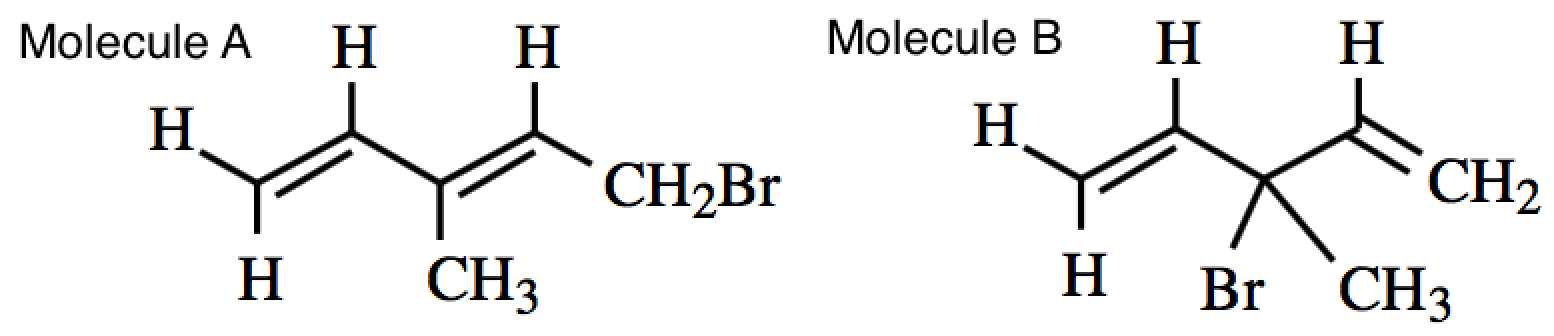 |
|
A
|
Molecule A is more stable because it has a more sterically hindered bromine.
|
B
|
Molecule A is more stable because it has conjugated double bonds.
|
C
|
Molecule B is more stable because it has a less sterically hindered bromine.
|
D
|
Molecule B is more stable because it has conjugated double bonds.
|
|
|
|
Tags:
Molecular Bonding | Molecular Structure | Organic Chemistry Reactions | |
|
| 11 |
Go |
Q:
|
The nitrogen-containing reactant below (Reactant B) can act as a nucleophile in certain reactions. The reaction below is carried out in a pH = 4.5 buffer. This is because the pKa of the protonated carbonyl (Reactant A) is between -2 and -8, so there is a low concentration of this highly reactive electrophile at a pH of 4.5. If the pH is further lowered (from 4.5) in order to try and increase the concentration of this electrophile, which of the following correctly describes the likely counter-effect?
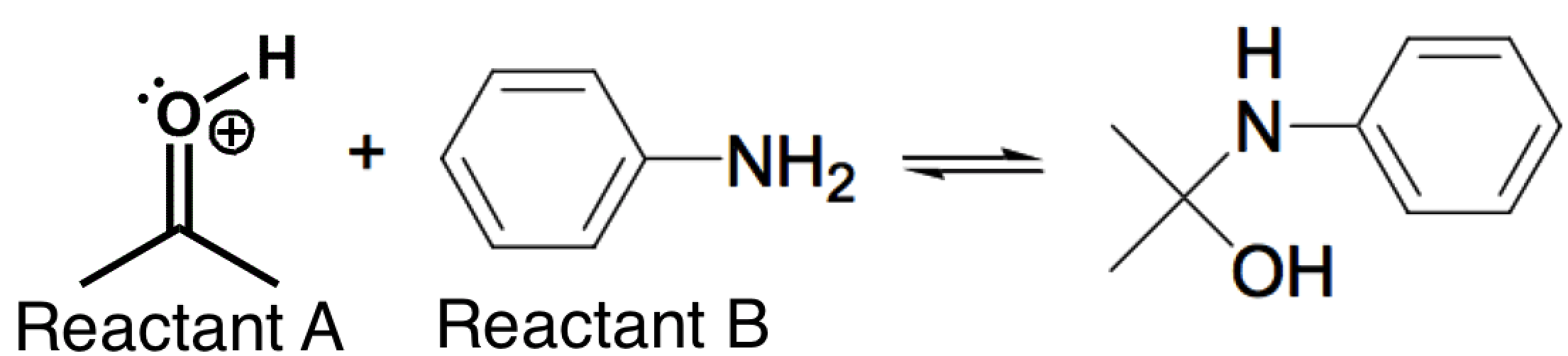 |
|
A
|
Increasing the acidity would cause less of Reactant B to become protonated (decreasing its nucleophilicity), and this would counteract the increase in electrophilicity of Reactant A.
|
B
|
Increasing the acidity would cause more of Reactant B to become protonated (decreasing its nucleophilicity), and this would counteract the increase in electrophilicity of Reactant A.
|
C
|
Increasing the acidity would cause more of Reactant B to become protonated and this would have no effect on the electrophilicity of Reactant A.
|
D
|
Increasing the acidity would have no effect on Reactant B and no effect on the electrophilicity of Reactant A.
|
|
|
|
Tags:
Acid/Base Equilibria | Miscellaneous Organic Chemistry | Organic Chemistry Reactions | Molecular Structure | |
|
| 12 |
Go |
Q:
|
Given the molecule below, which of the following is TRUE regarding its ability (or inability) to undergo an acylation reaction?
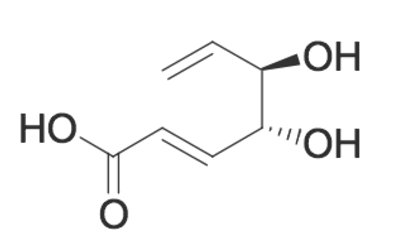 |
|
A
|
The molecule will undergo an intramolecular acylation reaction between the wedged alcohol and the carboxylic acid
|
B
|
The molecule will undergo an intramolecular acylation reaction between the dashed alcohol and the carboxylic acid
|
C
|
The molecule will NOT undergo an intramolecular acylation reaction because the double bond does not allow for the interaction between the alcohols and the carboxylic acid
|
D
|
The molecule will NOT undergo an intramolecular acylation reaction because acylation reactions cannot occur with carboxylic acids.
|
|
|
|
Tags:
Carboxylic Acids | Organic Chemistry Reactions | Molecular Structure | Alcohols | |
|
| 13 |
Go |
Q:
|
SN1 reactions are not stereospecific because: |
|
A
|
they involve a carbocation intermediate which can react to form one of two stereoisomers. |
B
|
they are attacked by strong nucelophiles. |
C
|
they have weak leaving groups. |
D
|
they require an ionic environment for stabilization. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 15 |
Go |
Q:
|
Otherwise lipid-soluble steroid molecules can be made water-soluble by which of the following modifications? |
|
A
|
reducing alcohol groups |
B
|
adding carboxylic acid groups |
C
|
oxidizing hydrogen atoms to alcohol groups |
D
|
decarboxylation |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 16 |
Go |
Q:
|
How does the oxidation state of carbon change when an alcohol is oxidized to an aldehyde? |
|
A
|
The oxidation state increases by 2. |
B
|
The oxidation state decreases by 2. |
C
|
The oxidation state increases by 1. |
D
|
The oxidation state decreases by 1. |
|
|
|
Tags:
Redox Reactions | Organic Chemistry Reactions | |
|
| 17 |
Go |
Q:
|
A nucleophilic reaction to a carbonyl is considered neither an SN1 nor SN2 reaction because: |
|
A
|
no substitution occurs during nucleophilic addition to a carbonyl. |
B
|
the reaction proceeds through an E1 or E2 mechanism instead. |
C
|
the solvent for the reaction is not aqueous. |
D
|
the solvent for the reaction is not polar. |
|
|
|
Tags:
Miscellaneous Organic Chemistry | Organic Chemistry Reactions | |
|
| 18 |
Go |
Q:
|
A nucleophilic functional group may also be thought of as a: |
|
A
|
Lewis acid. |
B
|
Lewis base. |
C
|
Bronsted acid. |
D
|
Bronsted base. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 19 |
Go |
Q:
|
The Tollens' test will yield a shiny silver mirror on the glass vial containing the reaction if what type of sugar is added? |
|
A
|
reducing sugar |
B
|
non-reducing sugar |
C
|
any disaccharide |
D
|
any non-monosaccharide |
|
|
|
Tags:
Carbohydrates | Organic Chemistry Reactions | |
|
| 20 |
Go |
Q:
|
Which of the following is FALSE regarding SN1 reactions? |
|
A
|
The second step is rate limiting. |
B
|
There are two steps in the reaction. |
C
|
The rate limiting step involves the departure of a leaving group. |
D
|
An intermediary carbocation is formed. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 21 |
Go |
Q:
|
Which of the following is a possible product of the reduction of an aldehyde? |
|
A
|
cyclohexanone |
B
|
butanol |
C
|
3-pentanol |
D
|
butyraldehyde |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 22 |
Go |
Q:
|
Ethanoyl chloride (CH3COCl) reacts with water to form a(n): |
|
A
|
carboxylic acid. |
B
|
ester. |
C
|
alcohol. |
D
|
amide. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 23 |
Go |
Q:
|
Which of the following is accurate regarding SN1 reactions? |
|
A
|
They take place in a single step. |
B
|
No nucleophilic attack is required. |
C
|
Halides very rarely serve as leaving groups. |
D
|
The reaction passes through a planar intermediate molecule. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 24 |
Go |
Q:
|
Various carbocations are produced in the laboratory with various groups attached to the carbon atom. Which of the following groups would yield least stable molecule? |
|
A
|
two ethyl groups and one methyl group |
B
|
all hydrogen atoms |
C
|
one ethyl group and two methyl groups |
D
|
three phenyl groups |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 25 |
Go |
Q:
|
An amide bond between amino acids can be formed via a: |
|
A
|
hydration reaction. |
B
|
dehydration reaction. |
C
|
decarboxylation reaction. |
D
|
redox reaction. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 26 |
Go |
Q:
|
Strecker synthesis is used in the synthesis of: |
|
A
|
fats. |
B
|
hydrocarbons. |
C
|
nucleic acids. |
D
|
amino acids. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 27 |
Go |
Q:
|
In organic reactions, hydrides are mostly utilized as: |
|
A
|
a stable R-group. |
B
|
reducing agents. |
C
|
largely inert. |
D
|
a proton-acceptor. |
|
|
|
Tags:
Organic Chemistry Reactions | |
|
| 28 |
Go |
Q:
|
Which of the following holds true regarding tautomerization? |
|
A
|
tautomers are molecules which differ in one or more stereocenters |
B
|
tautomers are defined as molecules differing in the placement of two or more atoms within a molecule |
C
|
equilibrium between tautomers rarely favors a single tautomer |
D
|
generally the keto form is more stable than the enol form in keto-enol tautomerization |
|
|
|
Tags:
Aldehydes and Ketones | Organic Chemistry Reactions | |
|
| 29 |
Go |
Q:
|
1,2-dimethylcyclopentene reacts with hydrochloric acid to form a compound. How many stereoisomers are created as products in this reaction? |
|
|
|
|
Tags:
Organic Chemistry Reactions | |
|
|
Questions? We're here to help!
Ask Us
|