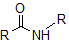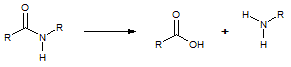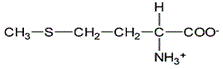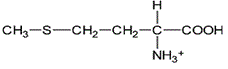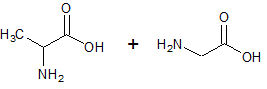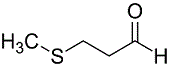|
| 2 |
Go |
Q:
|
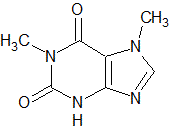
An unknown enzyme is isolated from human saliva. After heating it to 150 degrees C, which of the following is its most likely fate? |
|
A
|
Desaturation |
B
|
Denaturation |
C
|
Saturation |
D
|
Folding |
|
|
|
Tags:
Protein Structure and Function | |
|
| 3 |
Go |
Q:
|
Which of the following is likely to denature an enzyme?
I. Heating to 150 degrees C
II. Freezing to -20 degrees C
III. Freezing to -20 degrees C, then heating to 150 degrees C |
|
A
|
I only |
B
|
I and II only |
C
|
I and III only |
D
|
I, II, and III |
|
|
|
Tags:
Protein Structure and Function | |
|
| 4 |
Go |
Q:
|
Most proteins have optimal pH ranges. Once the pH shifts outside the optimal range, enzyme activity decreases.
The reason for this decrease is: |
|
A
|
a change in the 3-dimensional structure of the enzyme |
B
|
feedback inhibition |
C
|
allosteric inhibition |
D
|
decrease in the synthesis of the enzyme |
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 5 |
Go |
Q:
|
Chaotropic agents increase the solubility of nonpolar molecules in water. What effect is a chaotropic agent likely to have on tertiary protein structure? |
|
A
|
Strengthen the folding due to the increased solubility of the protein interior in water. |
B
|
Strengthen the folding due to the decreased solubility of the protein interior in water. |
C
|
Weaken the folding due to the increased solubility of the protein interior in water. |
D
|
Weaken the folding due to the decreased solubility of the protein in water. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 6 |
Go |
Q:
|
Sodium Dodecyl Sulfate (SDS) is a known denaturant but not a reducing agent. A protein sample is treated with SDS and is denatured. What is the effect of SDS on any disulfide bridges that may be present in the protein? |
|
A
|
The disulfide bridges are broken because SDS oxidizes the disulfide bridges. |
B
|
The disulfide bridges are broken because SDS reduces the disulfide bridges. |
C
|
The disulfide bridges are not broken because SDS strengthens disulfide bridges. |
D
|
The disulfide bridges are not broken because SDS does not affect disulfide bridges. |
|
|
|
Tags:
Protein Structure and Function | Intermolecular Forces | |
|
| 8 |
Go |
Q:
|
Which of the following would be least effective in rendering ATPase DnaK useless? |
|
A
|
Urea |
B
|
High concentration of Methane |
C
|
Acrylamide |
D
|
Very high temperature |
|
|
|
Tags:
Protein Structure and Function | |
|
| 10 |
Go |
Q:
|
Which of the following amino acids is a common target of protein kinases? |
|
A
|
threonine |
B
|
arginine |
C
|
tryptophan |
D
|
glutamic acid |
|
|
|
Tags:
Protein Structure and Function | |
|
| 11 |
Go |
Q:
|
In a peptide chain, the replacement of serine with
another amino acid would have the smallest impact on the
protein if the other amino acid were |
|
A
|
threonine |
B
|
glutamic acid |
C
|
proline |
D
|
tyrosine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 12 |
Go |
Q:
|
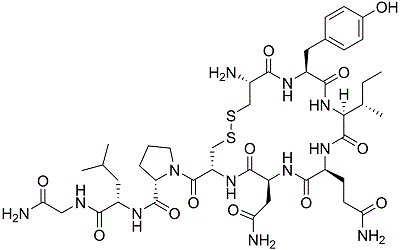
The following compound is biologically synthesized by amino acid derivatization.
From which amino acid(s) is the compound most likely synthesized?
|
|
A
|
tyrosine and tryptophan |
B
|
tryptophan and proline |
C
|
proline and phenylalanine |
D
|
two phenylalanines |
|
|
|
Tags:
Protein Structure and Function | |
|
| 13 |
Go |
Q:
|
Proteins are made from amino acids by the process of |
|
A
|
hydrolysis |
B
|
pinocytosis |
C
|
active transport |
D
|
dehydration synthesis |
|
|
|
Tags:
Protein Structure and Function | |
|
| 14 |
Go |
Q:
|
Which of the following amino acids contain one or more thiol groups? |
|
A
|
Methionine |
B
|
Threonine |
C
|
Histidine |
D
|
None of the above |
|
|
|
Tags:
Protein Structure and Function | |
|
| 15 |
Go |
Q:
|
Below are four sets of amino acids. All but one set of amino acids are structurally and physiologically similar. Identify the set of amino acids which contains an amino acid that is NOT structurally and physiologically similar to the others in its set. |
|
A
|
Valine, Alanine, Leucine |
B
|
Tyrosine, Phenylalanine, Histidine |
C
|
Serine, Threonine, Methionine |
D
|
Arginine, Asparagine, Glutamine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 18 |
Go |
Q:
|
F = kQq / εr2 where ε is the dielectric constant of a non-vacuum medium. Media of higher polarity have higher dielectric constants. Consider a protein in a solution of water. Two oppositely-charged amino acid residues of the protein are located 10 Angstroms apart, while a typical hydrogen bond has a length of 1.5-3.0 Angstroms. Which of the following scenarios would be predicted by the given information? Assume the distance between the amino acid residues and their respective charges remain constant in each scenario. |
|
A
|
The force pulling together the amino acid residues would increase if the residues were found on the surface of the protein than if they were found on the inside of the protein. |
B
|
The force pulling together the amino acid residues would decrease if the residues were found on the surface of the protein than if they were found on the inside of the protein. |
C
|
The force pulling together the amino acid residues would reverse direction (to push the residues apart) if the residues were found on the surface of the protein than if they were found on the inside of the protein. |
D
|
The force pulling together the amino acid residues would remain unchanged if the residues were found on the surface of the protein than if they were found on the inside of the protein. |
|
|
|
Tags:
Protein Structure and Function | Quantitative Skills | |
|
| 22 |
Go |
Q:
|
The α carbon of amino acids is the carbon |
|
A
|
to which the R group is attached |
B
|
that is always the chiral carbon of the amino acid |
C
|
that is found at the C terminus of proteins |
D
|
that forms the peptide bond with nitrogen |
|
|
|
Tags:
Protein Structure and Function | |
|
| 23 |
Go |
Q:
|
A polypeptide is typically translated from mRNA with 107 amino acids. After a mutation, the polypeptide truncates early after only 31 amino acids. Which type of mutation may have caused this result?
I. Frameshift Mutation
II. Point Mutation
III. Silent Mutation |
|
A
|
I only |
B
|
II only |
C
|
I and II only |
D
|
I, II, and III |
|
|
|
Tags:
Protein Structure and Function | Genetic Code, Transcription, Translation | |
|
| 24 |
Go |
Q:
|
A gene codes for an 84 amino acid polypeptide. A mutation renders the protein dysfunctional. Which of the following mutations is least likely to have such an impact? |
|
A
|
Frameshift mutation |
B
|
Silent mutation |
C
|
Deletion mutation |
D
|
Insertion mutation |
|
|
|
Tags:
Protein Structure and Function | |
|
| 25 |
Go |
Q:
|
Sodium Dodecyl Sulfate (SDS) is a reagent used in molecular biology to separate proteins based on molecular weight. SDS denatures secondary and non-disulfide-linked tertiary structures in a protein sample. Which of the following in a protein would be unaffected by SDS? |
|
A
|
Alpha helices |
B
|
Beta sheets |
C
|
Polypeptide bonds |
D
|
Dihedral Angles |
|
|
|
Tags:
Protein Structure and Function | |
|
| 26 |
Go |
Q:
|
Myoglobin is an oxygen-carrying protein very similar to hemoglobin in the way it binds oxygen. The protein, unlike hemoglobin, is composed of a single subunit. Based on this information, which of the following characteristics of hemoglobin does myoglobin not have? |
|
A
|
Ability to carry up to one oxygen molecule |
B
|
Cooperativity |
C
|
A heme group |
D
|
None of the above |
|
|
|
Tags:
Protein Structure and Function | |
|
| 27 |
Go |
Q:
|
Which of the following amino acid substitutions would likely have the greatest impact on the 3-dimensional structure of a protein? |
|
A
|
valine to leucine |
B
|
isoleucine to leucine |
C
|
tyrosine to phenylalanine |
D
|
aspartic acid to lysine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 28 |
Go |
Q:
|
Coomassie Brilliant Blue is a stain used in many biological applications. The stain binds along the length of protein polypeptides and can then be detected by the resulting blue color from the bound dye-protein complex. This information suggests that
I. A sample of proteins composed of 300 amino acids will produce a stronger stain than a sample of proteins composed of 200 amino acids, assuming the proteins are present in equimolar amounts
II. A 20 mM solution of a protein composed of 300 amino acids will produce a stronger stain than a 15 mM solution of the same protein
III. A 50 mM solution of proteins composed of 200 amino acids will stain stronger than a 25 mM solution of proteins composed of 400 amino acids. |
|
A
|
I only |
B
|
II only |
C
|
III only |
D
|
I and II only |
|
|
|
Tags:
Protein Structure and Function | |
|
| 30 |
Go |
Q:
|
Hemoglobin contains four subunits, each capable of binding oxygen. What effect does this have on the effectiveness of hemoglobin as an oxygen carrier? |
|
A
|
Allows for cooperativity in oxygen binding |
B
|
Hemoglobin is four times more efficient at carrying oxygen than one of its subunits |
C
|
Lower likelihood of mutations having a large impact on oxygen transport through the body |
D
|
The larger structure allows for slower movement, increasing time for oxygen pick-up and drop-off. |
|
|
|
Tags:
Protein Structure and Function | Circulatory System | |
|
| 32 |
Go |
Q:
|
A certain amino acid sequence on a protein intended for secretion from the cell was found to have a
role in transporting the protein into the rough ER. By splicing out this sequence, experimenters found
that the protein accumulated outside in the cytosol surrounding the rough ER (RER). No effect was found by
inserting this same sequence in another protein typically found in the cytosol. What can you say about
this amino acid sequence? |
|
A
|
It is necessary and sufficient for import into the RER. |
B
|
It is necessary but not sufficient for import into the RER. |
C
|
It is sufficient but not necessary for import into the RER. |
D
|
It is neither necessary nor sufficient for import into the RER. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 33 |
Go |
Q:
|
SDS is a negatively charged detergent used to disrupt noncovalent bonds between amino acids in a protein. One molecule of SDS binds on average to two residues of a protein. The protein segments are then separated by gel electrophoresis (an electric field is applied to the gel containing the protein segments) on the basis of molecular weight. The rate of movement of the protein through the gel is inversely proportional to the log of the molecular weight.
Which protein segments do you expect to be at the bottom of the gel after electrophoresis (furthest from the point of origin)?
|
|
A
|
The most negatively charged segments |
B
|
The heaviest segments |
C
|
The lightest segments |
D
|
The most positively charged segments |
|
|
|
Tags:
Protein Structure and Function | |
|
| 35 |
Go |
Q:
|
Chaperone proteins facilitate the formation of which protein structure? |
|
A
|
primary |
B
|
secondary |
C
|
tertiary |
D
|
none of the above |
|
|
|
Tags:
Protein Structure and Function | |
|
| 36 |
Go |
Q:
|
Which of the following amino acids is most likely to be found in the transmembrane domain of a protein? |
|
A
|
arginine |
B
|
glutamic acid |
C
|
asparagine |
D
|
valine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 38 |
Go |
Q:
|
A connective tissue protein is composed of multiple repeating protein subunits bound by noncovalent interactions. This type of structure is |
|
A
|
primary. |
B
|
secondary. |
C
|
tertiary. |
D
|
quaternary. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 39 |
Go |
Q:
|
A set of protein sequences are given in the tables below. Rank the proteins in increasing order of their ability to form disulfide bridges (covalent bonds between the R-groups of two cysteine sulfhydryl groups).
| Protein |
Sequence |
| 1 |
Alanine-Cysteine-Leucine-Threonine-Phenylalanine-Glutamine-Glycine-Glycine-Asparagine-Cysteine |
| 2 |
Cysteine-Cysteine-Leucine-Threonine-Phenylalanine-Glutamine-Glycine-Glycine-Asparagine-Glycine |
| 3 |
Alanine-Gylcine-Leucine-Threonine-Phenylalanine-Glutamine-Glycine-Glycine-Asparagine-Glycine |
|
|
A
|
I < II < III |
B
|
III < II < I |
C
|
II < III < I |
D
|
III < I < II |
|
|
|
Tags:
Protein Structure and Function | Intermolecular Forces | |
|
| 40 |
Go |
Q:
|
Transferring a protein from a 1 M electrolyte solution to a 0.1 M electrolyte solution would most likely have which of the following consequences for the protein? |
|
A
|
Protonating acidic residues while deprotonating basic residues |
B
|
Protonating acidic residues and protonating basic residues |
C
|
Deprotonating acidic residues and deprotonating basic residues |
D
|
Deprotonating acidic residues while protonating basic residues |
|
|
|
Tags:
Protein Structure and Function | |
|
| 41 |
Go |
Q:
|
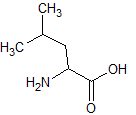
Leucine, the amino acid shown above, can be described as which of the following?
I. Amphipathic
II. Amphoteric
III. Aliphatic |
|
A
|
I and II only |
B
|
II and III only |
C
|
I and III only |
D
|
I, II, and III |
|
|
|
Tags:
Protein Structure and Function | |
|
| 42 |
Go |
Q:
|
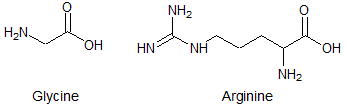
Polyglycine is a polypeptide composed of a string of glycine residues. It is a model polypeptide for many of its properties since glycine is the simplest amino acid. Compared to polyarginine composed of 20 residues, one can expect polyglycine composed of 20 residues to |
|
A
|
have a wider range of torsional angles due to decreased steric hindrance |
B
|
have a higher effective pKa |
C
|
form a more basic solution |
D
|
have a higher molecular weight |
|
|
|
Tags:
Protein Structure and Function | Molecular Structure | |
|
| 43 |
Go |
Q:
|
A missense mutation alters a leucine residue to an isoleucine residue, both similarly-sized nonpolar amino acids. The residue is typically found in the inside of an enzyme. This mutation is most likely to have which of the following effects? |
|
A
|
increase in the enzymatic activity |
B
|
slight decrease in the enzymatic activity |
C
|
dramatic decrease in the enzymatic activity |
D
|
markedly decreased resistance to changes in pH |
|
|
|
Tags:
Protein Structure and Function | |
|
| 44 |
Go |
Q:
|
Low concentrations of guanidinium hydrochloride, a strong denaturant, have been shown to convert prions (infectious, misfolded proteins with beta sheets) to non-prions (normal folding with alpha helices). Such a change involves a shift in: |
|
A
|
amino acid sequence on the protein. |
B
|
hydrogen bond arrangement of the peptide backbone. |
C
|
both ionic and hydrogen bond arrangement of the amino acid R-groups. |
D
|
sequence of the corresponding mRNA. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 45 |
Go |
Q:
|
Breaking of the peptide bond in proteins can be either chemically or enzymatically catalyzed in the presence of an acid. In both circumstances, there is a net addition of which of the following molecules? The peptide bond can be seen in the image below.
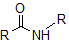 |
|
|
|
|
Tags:
Protein Structure and Function | |
|
| 46 |
Go |
Q:
|
Which of the following is the only class of biomolecules that contains sulfur? |
|
A
|
nucleic acid |
B
|
protein |
C
|
carbohydrate |
D
|
lipid |
|
|
|
Tags:
Protein Structure and Function | |
|
| 47 |
Go |
Q:
|
The R groups of the amino acids in α-helices ρ are directed outward from the helix, interacting with the surrounding space. The primary difference between a transmembrane alpha helix and a cytosolic alpha helix would be |
|
A
|
an abundance of nonpolar amino acids on the transmembrane helix versus an abundance of polar amino acids on the cytosolic helix. |
B
|
the presence of disulfides on the transmembrane helix but not on the cytosolic helix. |
C
|
long helices for transmembrane proteins and short helices for cytosolic proteins. |
D
|
a tighter turning radius for helices in transmembrane proteins than in cytosolic proteins. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 48 |
Go |
Q:
|
All amino acids have at least two pKa values. Which of the following functional groups would provide an amino acid a third pKa value (between 0 and 14)? |
|
A
|
-CH2CH2CONH2 |
B
|
-C(OH)CH3 |
C
|
-CH2CH2SCH3 |
D
|
-CH2CH2CH2CH2NH2 |
|
|
|
Tags:
Protein Structure and Function | |
|
| 50 |
Go |
Q:
|
SDS is a negatively charged detergent used to disrupt noncovalent bonds between amino acids in a protein. One molecule of SDS binds on average to two residues of a protein. The protein segments are then separated by gel electrophoresis (an electric field is applied to the gel containing the protein segments) on the basis of molecular weight. The rate of movement of the protein through the gel is inversely proportional to the log of the molecular weight.
The separation of nucleic acids by electrophoresis does not require SDS. Why?
|
|
A
|
There are no residue groups on nucleic acids to which SDS can bind |
B
|
Nucleic acids can separate based on their hydrophobicity without the binding of SDS |
C
|
Nucleic acids have phosphate groups that contribute net negative charges proportional to their size |
D
|
Nucleic acids do not have tertiary structures like protein that need to be disrupted |
|
|
|
Tags:
Protein Structure and Function | Nucleic Acid Structure and Function | |
|
| 51 |
Go |
Q:
|
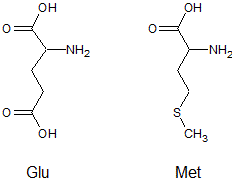
At a pH of 13, what is the expected difference in charge between the amino acids glutamic acid (Glu) and methionine (Met)?
 |
|
A
|
Glu and Met have the same charge |
B
|
Glu has a charge 1 unit greater than Met |
C
|
Glu has a charge 2 units less than Met |
D
|
Glu has a charge 1 unit less than Met |
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 52 |
Go |
Q:
|
Which of the following statements is FALSE regarding the primary and secondary amines? |
|
A
|
Primary amines are in general stronger bases than secondary amines. |
B
|
Primary amines are less sterically hindered than secondary amines. |
C
|
Primary and secondary amines both have a lone pair of electrons on the nitrogen atom. |
D
|
Primary and secondary amines can both be used in the production of an amide. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 53 |
Go |
Q:
|
Proteases degrade proteins by breaking peptide bonds. In the absence of proteases, protein peptide bonds can be broken by which of the following methods? |
|
A
|
addition of an oxidizing agent like H2O2 |
B
|
dehydrogenation |
C
|
placing the proteins in water; peptide bonds will hydrolyze in water under normal conditions |
D
|
acid hydrolysis |
|
|
|
Tags:
Protein Structure and Function | |
|
| 54 |
Go |
Q:
|
Which of the following is NOT correct in regard to alanine and amino acid catabolism?
|
|
A
|
Alanine cannot be formed from the initial breakdown of glucose during glycolysis
|
B
|
Alanine is utilized and transported to the liver (from the muscles) during amino acid catabolism
|
C
|
Alanine is formed from pyruvate in the muscles
|
D
|
Alanine's reconstitution in the liver results in the production of urea
|
|
|
|
Tags:
Protein Structure and Function | Metabolism of Fatty Acids and Proteins | Glycolysis, Gluconeogenesis, PPP | |
|
| 55 |
Go |
Q:
|
Proteins distributed on a pH gradient and separated by their isoelectric point will be located at which position? |
|
A
|
The point where the protein has denatured and is linear. |
B
|
The point where the protein's size corresponds to the pore size of the gel matrix. |
C
|
The point where the protein has been completely deprotonated. |
D
|
The point where the protein's pKa is equal to the external pH. |
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 56 |
Go |
Q:
|
Which of the following, if true, casts doubt on the assertion that tryptophan in turkey meat is responsible for induction of drowsiness after consumption? |
|
A
|
Other commonly-consumed meats like chicken and pork have higher concentrations of tryptophan than turkey. |
B
|
Large meals containing high amounts of turkey meat but lacking high amounts of carbohydrates do not induce drowsiness. |
C
|
Large meals containing high amounts of carbohydrates but lacking turkey meat induce drowsiness. |
D
|
Consumption of pure tryptophan causes drowsiness. |
|
|
|
Tags:
Protein Structure and Function | Quantitative Skills | |
|
| 58 |
Go |
Q:
|
The large number of potential conformations of polypeptides is due largely to which of the following? |
|
A
|
C-C bonds have a large degree of rotational freedom |
B
|
disulfide bridges add strength to a host of different conformations |
C
|
hydrogen bonds make polypeptide conformations stronger |
D
|
C-C-H bonds can flex easily |
|
|
|
Tags:
Protein Structure and Function | |
|
| 59 |
Go |
Q:
|
The pungent smell of burning hair is due to the large concentration of sulfur in keratin. Which of the following describes the role of the sulfur? |
|
A
|
It is concentrated in hair along with other environmental toxins. |
B
|
It is present in disulfide bridges in keratin. |
C
|
It is part of the carbohydrate structure of the hair. |
D
|
It acts as a pigment for the keratin in the hair. |
|
|
|
Tags:
Protein Structure and Function | Intermolecular Forces | |
|
| 60 |
Go |
Q:
|
Scientists have identified the compound tryptophol to be a derivative of the amino acid tryptophan. Tryptophol induces drowsiness in humans, and a student concludes based on this information that consumption of tryptophan will induce sleep in humans through the production of tryptophol. Is the student correct in this conclusion, and why or why not? |
|
A
|
Yes, because tryptophol is a derivative of tryptophan and therefore it will be produced when tryptophan is consumed. |
B
|
Yes, because tryptophol is produced regardless of the presence of tryptophan. |
C
|
No, because humans tryptophan is not absorbed and will be excreted regardless of the production of tryptophol. |
D
|
No, because humans might lack the necessary enzymes to catalyze the conversion of tryptophan to tryptophol. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 61 |
Go |
Q:
|
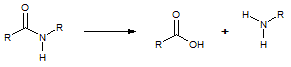
The reaction that proteases perform which breaks the peptide bond as in the reaction scheme above is known as: |
|
A
|
condensation |
B
|
hydrogenation |
C
|
tautomerization |
D
|
hydrolysis |
|
|
|
Tags:
Protein Structure and Function | |
|
| 62 |
Go |
Q:
|
Protein electrophoresis generally uses denatured proteins to compare which of the following attributes? |
|
A
|
length of primary sequence |
B
|
degree of bonding in secondary structure |
C
|
degree of folding in tertiary structure |
D
|
number of subunits in quaternary sturcture |
|
|
|
Tags:
Protein Structure and Function | |
|
| 63 |
Go |
Q:
|
Unlike enzymatic amino acid synthesis, most chemical amino acid synthesis produces racemic mixtures. The difference is primarily due to which of the following? |
|
A
|
Enzymes work at lower temperatures and are therefore able to catalyze more sensitive biochemical reactions. |
B
|
Enzymatic reactions are usually stereospecific. |
C
|
Chemical synthesis involves reagents that are racemic. |
D
|
Chemical synthesis has a higher activation energy making it more likely to produce a racemic mixture. |
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 64 |
Go |
Q:
|
Gout is a type of arthritis that can be caused by a high dietary intake of purines. Individuals seeking to avoid dietary purines should avoid consumption of food with high proportions of which of the following? |
|
A
|
proteins |
B
|
fats |
C
|
carbohydrates |
D
|
nucleic acids |
|
|
|
Tags:
Protein Structure and Function | |
|
| 66 |
Go |
Q:
|
The reaction of the two amino acids below to form a peptide bond is an example of which of the following types of reactions?
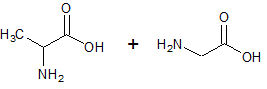 |
|
A
|
hydrolysis |
B
|
condensation |
C
|
esterification |
D
|
decarboxylation |
|
|
|
Tags:
Protein Structure and Function | |
|
| 67 |
Go |
Q:
|
When the tertiary structure of a protein is disrupted, the secondary structure of the protein is usually also disrupted. This is primarily because: |
|
A
|
tertiary structure holds together the secondary structure. |
B
|
secondary structure is composed of tertiary structure. |
C
|
disruption of any structural aspect of proteins destroys all structural aspects of proteins. |
D
|
secondary and tertiary structure are held together by similar intermolecular bonds. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 68 |
Go |
Q:
|
Which of the following is an inaccuracy regarding peptide bonds and polypeptide structure? |
|
A
|
Polypeptides are formed through a condensation reaction between two amino acids. |
B
|
At times, the peptide bond is composed of disulfide bond, as in the case of cysteine. |
C
|
Peptide bonds with a polypeptide are broken through a hydrolysis reaction. |
D
|
The carboxyl-carbon on one amino acid acts as the electrophile the hydrolysis reaction. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 69 |
Go |
Q:
|
At the isoelectric point of a protein, which of the following is TRUE? |
|
A
|
There is no charge on the protein |
B
|
The number of positive charges is equal to the number of negative charges on the protein |
C
|
The protein is nonpolar |
D
|
The protein has the same charge that it does at a pH of 7 |
|
|
|
Tags:
Protein Structure and Function | |
|
| 70 |
Go |
Q:
|
Which of the following is false regarding amino acids? |
|
A
|
They are zwitterionic |
B
|
They all have an (R) and an (S) configuration |
C
|
They make up the primary structure of proteins |
D
|
They are carried by tRNA molecules |
|
|
|
Tags:
Protein Structure and Function | |
|
| 71 |
Go |
Q:
|
α-helices are integral to which of the following structures within the body? |
|
A
|
Cytosolic proteins involved in degradation of genomic material |
B
|
Cytosolic, non-enzymatic proteins |
C
|
The support structure around DNA |
D
|
Specialized proteins fixed within the cell membrane |
|
|
|
Tags:
Protein Structure and Function | Membrane Transport and Signalling | |
|
| 72 |
Go |
Q:
|
Which of the following amino acid substitutions would be most disruptive to protein structure? |
|
A
|
Aspartic acid to Glutamic acid |
B
|
Lysine to Arginine |
C
|
Glycine to Tryptophan |
D
|
Leucine to Isoleucine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 73 |
Go |
Q:
|
A newly discovered protein is studied in the laboratory and called dorminogen. Based on the nomenclature of proteins, it can be assumed that this protein: |
|
A
|
is a neurotransmitter. |
B
|
cleaves other proteins. |
C
|
is an intranuclear protein. |
D
|
is a precursor protein. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 74 |
Go |
Q:
|
A Drosophila (fruit fly) protein is studied in the laboratory and it is discovered that the tertiary structure of this peptide has a large number of tight turns such that the individual peptides change direction frequently. Which amino acid would be expected to have a high frequency within this protein? |
|
A
|
proline |
B
|
glycine |
C
|
lysine |
D
|
valine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 75 |
Go |
Q:
|
Which of the following correctly describes the two functional groups involved in the formation of a peptide bond? |
|
A
|
Amino, carbonyl |
B
|
Amino, carboxyl |
C
|
Acetal, carboxyl |
D
|
Acetyl, glycosyl |
|
|
|
Tags:
Protein Structure and Function | |
|
| 77 |
Go |
Q:
|
Three solutions are mixed, each with a high concentration of amino acids. Solution A has a high concentration of basic amino acids, Solution B has a high concentration of acidic amino acids, and Solution C is nearly all non-polar amino acids. Using isoelectric focusing as an electrophoresis method to distinguish these proteins, and given that the bottom of the gel is toward a lower pH, which of the following lists (from bottom to top) the positions of these proteins as a result of the separation? |
|
A
|
Basic proteins, non-polar proteins, acidic proteins
|
B
|
Non-polar proteins, acidic proteins, basic proteins
|
C
|
Acidic proteins, basic proteins, non-polar proteins
|
D
|
Acidic proteins, non-polar proteins, basic proteins
|
|
|
|
Tags:
Protein Structure and Function | Separation and Purification | |
|
| 78 |
Go |
Q:
|
The preferred food source for E. coli is glucose, but in the absence of glucose, E. coli can metabolize other sugar sources, like tremalose. The tremalose operon is regulated by a repressor protein which ONLY binds to the operator in the absence of tremalose. Moreover, the operon is also regulated by an activator which ONLY binds to the promoter (where RNA polymerase binds) in the absence of glucose. Binding of this activator is needed for any expression of tremalose operon genes. Which of the following correctly describes the conditions under which the tremalose operon will be expressed? |
|
A
|
In the presence of BOTH glucose and tremalose
|
B
|
In the absence of BOTH glucose and tremalose
|
C
|
In the presence of glucose and the absence of tremalose
|
D
|
In the absence of glucose and the presence of tremalose
|
|
|
|
Tags:
Control of Gene Expression in Prokaryotes | Protein Structure and Function | |
|
| 79 |
Go |
Q:
|
Which of the following correctly describes the interactions present in the quaternary structure of a protein? |
|
A
|
Peptide bonds only
|
B
|
Hydrogen bonds only
|
C
|
Hydrogen bonds, ionic bonds, and phosphodiester bonds
|
D
|
Hydrogen bonds, ionic bonds, and Van der Waals interactions
|
|
|
|
Tags:
Protein Structure and Function | |
|
| 80 |
Go |
Q:
|
Which of the following is true of carrier proteins that are used in facilitated diffusion? |
|
A
|
Carrier proteins used in facilitated diffusion are usually coupled to ATP hydrolysis
|
B
|
Carrier proteins used in facilitated diffusion are specific for a specific type of molecule
|
C
|
Carrier proteins used in facilitated diffusion are located in the nuclear membrane
|
D
|
Carrier proteins used in facilitated diffusion work against concentration gradients
|
|
|
|
Tags:
Protein Structure and Function | Membrane Transport and Signalling | |
|
| 81 |
Go |
Q:
|
In proteins, secondary structure is due to which type of interactions? |
|
A
|
Hydrogen bonds between the side chains of amino acids
|
B
|
Hydrogen bonds between sections of the polypeptide backbone
|
C
|
Peptide bonds between adjacent amino acids
|
D
|
Peptide bonds between cysteine amino acids
|
|
|
|
Tags:
Protein Structure and Function | |
|
| 82 |
Go |
Q:
|
Lab results from a protein analysis reveal its complete structure, and that it catalyzes the breakdown of a larger substrate. Moreover, there are two binding sites (one for the large substrate, and one for a small regulatory molecule). Which of the following best describes the mechanism by which this protein works? |
|
A
|
The protein is likely structural, and is involved in cell-to-cell adhesion
|
B
|
The protein is likely an enzyme that works through competitive inhibition
|
C
|
The protein is likely an enzyme that works through allosteric regulation
|
D
|
The protein is likely a membrane transport protein for ions
|
|
|
|
Tags:
Protein Structure and Function | Enzyme Structure and Function | |
|
| 83 |
Go |
Q:
|
A step in the lab procedure for a protein analysis involves placing cytoplasmic protein in a hydrocarbon (e.g. hexane). Which of the following would most likely NOT happen next? |
|
A
|
The protein would fold itself into a different conformation
|
B
|
The protein would retain the conformation it had prior to entering the hydrocarbon solution
|
C
|
The polar side-groups would orient themselves away from hydrocarbon
|
D
|
The nonpolar side-groups would interact with the hydrocarbon molecules via Van der Waals interactions
|
|
|
|
Tags:
Protein Structure and Function | Separation and Purification | |
|
| 84 |
Go |
Q:
|
A recently polymerized protein is still linear. What is the highest level of structure that can be attributed to this protein? |
|
A
|
Primary |
B
|
Secondary |
C
|
Tertiary |
D
|
Quaternary |
|
|
|
Tags:
Protein Structure and Function | |
|
| 85 |
Go |
Q:
|
Similar to those found in carbohydrates, which type of protein linkage is most similar to a glycosidic linkage? |
|
A
|
Alpha helix
|
B
|
Beta sheet |
C
|
Disulfide bond
|
D
|
Peptide bond |
|
|
|
Tags:
Protein Structure and Function | Carbohydrates | |
|
| 86 |
Go |
Q:
|
A protein found in mice is sequenced and the results show sulfur-containing cysteine residues occur at regularly-spaced intervals. Based on these results, which of the following best describes the significance and function of these residues? |
|
A
|
Cysteine amino acids are required for the formation of alpha-helices
|
B
|
Sulfur must be an important co-factor
|
C
|
Cysteine amino acids form a hydrophobic core in the protein
|
D
|
Cysteine amino acids are involved in disulfide bridges that help form tertiary structure
|
|
|
|
Tags:
Protein Structure and Function | |
|
| 87 |
Go |
Q:
|
Which of the following is correct in regard to the most common type of hemoglobin in normal adults? |
|
A
|
One molecule of hemoglobin contains 1 atom of iron |
B
|
One molecule of hemoglobin contains 4 atoms of iron |
C
|
The binding of oxygen is a non-cooperative process |
D
|
The the oxygen binding curve of hemoglobin results in a hyperbolic curve |
|
|
|
Tags:
Protein Structure and Function | Circulatory System | |
|
| 88 |
Go |
Q:
|
Which of the following best describes the concept of an amphipathic molecule?
|
|
A
|
An amphipathic molecule has one positively charged region and one negatively charged region
|
B
|
An amphipathic molecule is branched, and with at least two branch points
|
C
|
An amphipathic molecule has one polar region and one nonpolar region
|
D
|
An amphipathic molecule has two different types of bonds
|
|
|
|
Tags:
Protein Structure and Function | Lipids | Membrane Transport and Signalling | |
|
| 89 |
Go |
Q:
|
All individual amino acids are soluble in water; however, not all peptides are soluble. Which of the following choices best explains this? |
|
A
|
The R groups of the amino acid residues in the peptide are charged at physiological pHs
|
B
|
The R groups on all amino acids can interact noncovalently with water at pH = 7.4 (the pH of a normal, healthy body)
|
C
|
All peptides are insoluble in water
|
D
|
Individual amino acids are zwitterions at physiological pHs
|
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 90 |
Go |
Q:
|
Which of the following techniques is used to separate cellular proteins and is characterized by proteins leaving the mobile phase and associating with a negatively charged immobile substrate? |
|
A
|
Cation exchange chromatography
|
B
|
Anion exchange chromatography
|
C
|
Affinity chromatography
|
D
|
Micellar liquid chromatography
|
|
|
|
Tags:
Separation and Purification | Protein Structure and Function | |
|
| 91 |
Go |
Q:
|
In untreated diabetics, excess glucose in the blood is due to incomplete reabsorption (normally done by transporters present in the nephrons of the kidney) and subsequent excretion in the urine. Which of the following best describes this effect?
|
|
A
|
The transporters are inhibited by high levels of glucose
|
B
|
The Vmax of the transporter is reached at lower blood glucose levels
|
C
|
The binding affinity of these transporters is increased in diabetics
|
D
|
The binding capacity of the transporters has been exceeded
|
|
|
|
Tags:
Membrane Transport and Signalling | Renal System | Protein Structure and Function | Carbohydrates | |
|
| 92 |
Go |
Q:
|
Which of the following is NOT true about insulin? |
|
A
|
Insulin increases the intracellular concentration of glucose in hepatocytes |
B
|
Insulin increases the capacity of the liver to synthesize glycogen |
C
|
Insulin has effects opposite to the effects of glucagon |
D
|
Insulin has effects similar to the effects of epinephrine |
|
|
|
Tags:
Hormonal Regulation | Protein Structure and Function | |
|
| 98 |
Go |
Q:
|
In skeletal muscle contraction, which of the following provides the most important source of calcium ions? |
|
A
|
Triphosphate binding to a receptor on the sarcoplasmic reticulum membrane.
|
B
|
The opening of calcium channels in the sarcoplasmic reticulum.
|
C
|
The calcium ATPase in the sarcoplasm.
|
D
|
Voltage-gated calcium channels in the muscle fiber plasma membrane.
|
|
|
|
Tags:
Protein Structure and Function | Musculoskeletal System | Membrane Transport and Signalling | |
|
| 99 |
Go |
Q:
|
Amino acids are characterized by two highly versatile functional groups. Which of the following is NOT a reaction that amino acids can undergo due to these functional groups? |
|
A
|
Nucleophilic addition |
B
|
Esterification |
C
|
Amide bond formation |
D
|
Keto-enol tautomerism |
|
|
|
Tags:
Carboxylic Acids | Protein Structure and Function | Aldehydes and Ketones | |
|
| 100 |
Go |
Q:
|
Which of the following would NOT effectively separate amino acids and fatty acids? |
|
A
|
Separating on the basis of the properties (charge and binding affinities) of amino groups.
|
B
|
Separating based on the reactivity of any carboxylic acid group present.
|
C
|
Separating in water on the basis of solubility differences.
|
D
|
Separating the two based on the difference in size and shape.
|
|
|
|
Tags:
Lipids | Separation and Purification | Protein Structure and Function | |
|
| 105 |
Go |
Q:
|
Which of the following is true of the major histocompatibility complex (MHC)? |
|
A
|
Four classes of the major histocompatibility complex exist.
|
B
|
T cells do not detect infections through MHC.
|
C
|
MHC I acts as a signal for viruses.
|
D
|
MHC molecules display protein fragments from a pathogen for recognition by B cells.
|
|
|
|
Tags:
Protein Structure and Function | Immune System | Membrane Transport and Signalling | |
|
| 107 |
Go |
Q:
|
Ammonium sulfate precipitation involves the addition of the highly-soluble salt ammonium sulfate to raise the ion concentration until proteins differentially precipitate based on their solubility at high ion concentrations. This technique is most similar to: |
|
A
|
mass spectrometry. |
B
|
column chromatography. |
C
|
electrophoresis. |
D
|
PCR. |
|
|
|
Tags:
Chemistry Laboratory Techniques | Protein Structure and Function | |
|
| 108 |
Go |
Q:
|
A newly-identified protein is noted for containing no sulfur. It therefore must lack: |
|
A
|
tryptophan |
B
|
cysteine |
C
|
histidine |
D
|
arginine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 109 |
Go |
Q:
|
An ultra-conserved element is a genetic sequence that does not change in at least two different species, often in distantly-related species. Where are these elements most likely to be found? |
|
A
|
catalytic regions of metabolic enzymes |
B
|
binding regions cell surface proteins |
C
|
DNA-binding regions of transcription factors |
D
|
non-catalytic regions of digestive enzymes |
|
|
|
Tags:
Protein Structure and Function | Evolution | |
|
| 110 |
Go |
Q:
|
The amide bond is a key component of which of the following? |
|
A
|
proteins |
B
|
carbohydrates |
C
|
nucleic acids |
D
|
lipids |
|
|
|
Tags:
Protein Structure and Function | |
|
| 111 |
Go |
Q:
|
A protein readily soluble in water with a pI of 3.6 has predominantly what type of group on its surface? |
|
A
|
aromatic |
B
|
acidic |
C
|
hydrophobic |
D
|
basic |
|
|
|
Tags:
Protein Structure and Function | |
|
| 112 |
Go |
Q:
|
Which of the following best explains why glutamic acid is less soluble than glycine in water at a neutral pH? |
|
A
|
Glutamic acid is more acidic. |
B
|
Glutamic acid does not dissociate in water. |
C
|
Glutamic acid is larger and more hydrophobic than glycine. |
D
|
Glutamic acid has more nitrogen atoms. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 113 |
Go |
Q:
|
Which amino acid substitution would be most disruptive to the structure of an alpha helix? |
|
A
|
Glu to Tyr |
B
|
Arg to Trp |
C
|
Lys to Pro |
D
|
Gly to Ile |
|
|
|
Tags:
Protein Structure and Function | |
|
| 114 |
Go |
Q:
|
Which of the following is false regarding a right-shift of the oxygen-hemoglobin dissociation curve? |
|
A
|
It reflects decreased affinity of hemoglobin for oxygen. |
B
|
It is often times referred to as the Bohr effect. |
C
|
It can be caused by increased temperature. |
D
|
It can be caused by increased pH. |
|
|
|
Tags:
Circulatory System | Protein Structure and Function | |
|
| 115 |
Go |
Q:
|
Which pair of amino acids is structurally very similar but has very different biochemical properties? |
|
A
|
leucine and isoleucine |
B
|
glutamine and gluatmic acid |
C
|
glycine and proline |
D
|
arginine and tryptophan |
|
|
|
Tags:
Protein Structure and Function | |
|
| 117 |
Go |
Q:
|
Which of the following amino acids would have the most restrictive torsional angles? |
|
A
|
methionine |
B
|
proline |
C
|
glycine |
D
|
arginine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 119 |
Go |
Q:
|
Which peptide sequence would be LEAST likely to be found in a transmembrane space? |
|
A
|
PKSRK |
B
|
WGVLI |
C
|
FWMFA |
D
|
GGAVA |
|
|
|
Tags:
Protein Structure and Function | |
|
| 121 |
Go |
Q:
|
Which of the following is least contributory to tertiary structure of a polypeptide? |
|
A
|
disulfide bridges between regions of the polypeptide sequence |
B
|
hydrophobic side chains |
C
|
Van der Waals forces between various regions of the polypeptide molecule |
D
|
peptide bonding that links adjacent amino acids (namely single bonds between carbon and nitrogen) |
|
|
|
Tags:
Protein Structure and Function | |
|
| 122 |
Go |
Q:
|
Which of the following correctly identifies an amino acid with a hydrophobic side chain? |
|
A
|
isoleucine |
B
|
serine |
C
|
aspartate |
D
|
histidine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 123 |
Go |
Q:
|
Which of the following amino acids does NOT have a third titration point? |
|
A
|
phenylalanine |
B
|
aspartic acid |
C
|
glutamic acid |
D
|
lysine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 125 |
Go |
Q:
|
Even if the amino acid sequence of a particular protein is identical between two different species, the proteins can typically be differentiated by: |
|
A
|
the presence and concentration of Carbon-14. |
B
|
the glycosylation patterns on the proteins. |
C
|
the DNA labels on the proteins. |
D
|
the pI of the proteins. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 126 |
Go |
Q:
|
The linkage of two peptides via a peptide bond is what type of reaction? |
|
A
|
dehydrogenation |
B
|
dehydration |
C
|
hydrolytic |
D
|
decarboxylation |
|
|
|
Tags:
Protein Structure and Function | |
|
| 127 |
Go |
Q:
|
Which of the following amino acids does NOT have a polar side chain? |
|
A
|
serine |
B
|
leucine |
C
|
threonine |
D
|
asparagine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 128 |
Go |
Q:
|
Alpha helices serve as a examples of which type of protein structure? |
|
A
|
Primary |
B
|
Secondary |
C
|
Tertiary |
D
|
Quaternary |
|
|
|
Tags:
Protein Structure and Function | |
|
| 129 |
Go |
Q:
|
The transmembrane portion of a particular membrane protein likely contains which of the following amino acids? |
|
A
|
tyrosine |
B
|
serine |
C
|
glutamate |
D
|
methionine |
|
|
|
Tags:
Protein Structure and Function | |
|
| 130 |
Go |
Q:
|
Microscopically, it is found that water molecules arrange in a neat, orderly pattern around a cation molecule under study. This pattern describes a: |
|
A
|
ionic bonding complex. |
B
|
covalent bond. |
C
|
aqueous repulsion. |
D
|
solvation shell. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 133 |
Go |
Q:
|
Which of the following is an interaction which contributes to secondary structure of a protein? |
|
A
|
hydrogen bonds between R-groups on adjacent amino acids |
B
|
hydrogen bonds between two different peptide molecules |
C
|
hydrogen bonds between adjacent hydrogens on a peptide backbone |
D
|
nucleotide interactions between two separate peptide molecules |
|
|
|
Tags:
Protein Structure and Function | |
|
| 134 |
Go |
Q:
|
The Bohr effect provides explanation for: |
|
A
|
increased hemoglobin affinity for oxygen at higher temperatures. |
B
|
increased respiratory rate in response to low oxygen states. |
C
|
increased oxygen delivery to exercising muscle. |
D
|
increased affinity of hemoglobin for oxygen the more oxygen molecules that bind to hemoglobin. |
|
|
|
Tags:
Protein Structure and Function | Respiratory System | |
|
| 135 |
Go |
Q:
|
The secondary structure of proteins is mostly due to: |
|
A
|
van der Waals forces. |
B
|
covalent bonding between "R" groups. |
C
|
hydrogen bonding. |
D
|
the number of amino acids. |
|
|
|
Tags:
Protein Structure and Function | |
|
| 136 |
Go |
Q:
|
Which naturally occurring L-amino acid within the human body is the only one to exist as an (R) configuration?
|
|
A
|
Lysine
|
B
|
Cysteine
|
C
|
Glycine
|
D
|
Tryptophan
|
|
|
|
Tags:
Protein Structure and Function | |
|
|
Questions? We're here to help!
Ask Us
|