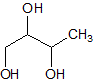
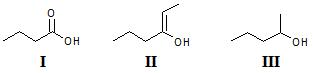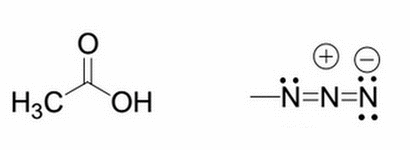|
| 2 |
Go |
Q:
|
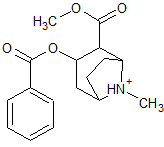
The following molecule contains all of which list of functional groups?
 |
|
A
|
carboxylic acid, ester |
B
|
alcohol, ketone, ester |
C
|
carboxylic acid, ether, ketone |
D
|
alcohol, ketone, ether |
|
|
|
Tags:
Atomic & Electronic Structure | Carboxylic Acids | |
|
| 4 |
Go |
Q:
|
Decarboxylation involves the removal of a carboxyl group and its replacement with a hydrogen. The carboxyl group is removed as |
|
A
|
formaldehyde |
B
|
carbon dioxide |
C
|
carbon monoxide |
D
|
formic acid |
|
|
|
Tags:
Carboxylic Acids | |
|
| 5 |
Go |
Q:
|
Which class of compounds has the general formula R-COO-R'?
|
|
A
|
Ether |
B
|
Peroxide |
C
|
Ester |
D
|
Ketone |
|
|
|
Tags:
Carboxylic Acids | |
|
| 6 |
Go |
Q:
|
Many mainly-nonpolar carboxylic acids with long alkyl chains are insoluble in water when protonated. With which of the following can they be reacted in order to become soluble? |
|
A
|
HCl |
B
|
NaCl |
C
|
MeOH |
D
|
NaOH |
|
|
|
Tags:
Carboxylic Acids | Redox Reactions | Intermolecular Forces | |
|
| 7 |
Go |
Q:
|
Carboxylic acids can be formed by the
I. Oxidation of alcohols
II. Oxidation of aldehydes
III. Reduction of ketones |
|
A
|
I and III only |
B
|
I and II only |
C
|
II and III only |
D
|
I, II, and III |
|
|
|
Tags:
Carboxylic Acids | |
|
| 8 |
Go |
Q:
|
XCH2COOH
In the compound above, which of the following at "X" would result in the weakest carboxylic acid species? |
|
|
|
|
Tags:
Carboxylic Acids | |
|
| 10 |
Go |
Q:
|
A carboxylic acid anhydride and hydrochloric acid are produced from a reaction between which two reactants? |
|
A
|
water and acid chloride |
B
|
acid chloride and RCOOH |
C
|
Cl2 and ROH |
D
|
Cl2 and RNH2 |
|
|
|
Tags:
Carboxylic Acids | |
|
| 11 |
Go |
Q:
|
The molecule with the formula C7H14O2 could be which of the following types of compounds?
I. Ester
II. Carboxylic Acid
III. Alcohol (diol) |
|
A
|
I and II only |
B
|
I and III only |
C
|
II and III only |
D
|
I, II, and III |
|
|
|
Tags:
Alcohols | Carboxylic Acids | |
|
| 12 |
Go |
Q:
|
During a purification experiment, Compound X is extracted from ethyl acetate in which it is readily soluble into an aqueous Solution A in which it is only moderately soluble. Compound X has a pKa of 2.8 and has a carboxylic acid as its primary functional group. In the next step, the student wants to precipitate Compound X and attempts different experimental conditions. The observations from two conditions are shown in the table below.
| � |
Condition 1 |
Condition 2 |
| pH |
6.5 |
0.5 |
Assuming the there is no difference in the experimental conditions except for the pH, which of the following is true? |
|
A
|
Condition 1 will precipitate Compound X because Compound X will be insoluble when the pH is above the pKa |
B
|
Condition 2 will precipitate Compound X because Compound X will be insoluble when the pH is below the pKa |
C
|
Condition 1 will precipitate Compound X because Compound X will be insoluble when the pH is below the pKa |
D
|
Condition 2 will precipitate Compound X because Compound X will be insoluble when the pH is above the pKa |
|
|
|
Tags:
Acid/Base Equilibria | Solutions | Carboxylic Acids | |
|
| 13 |
Go |
Q:
|
In the decarboxylation reaction of butanoic acid, the molecular weight of the resulting product will be |
|
A
|
44 g/mol |
B
|
71 g/mol |
C
|
72 g/mol |
D
|
87 g/mol |
|
|
|
Tags:
Carboxylic Acids | |
|
| 14 |
Go |
Q:
|
Carboxyl groups have higher boiling points than acid halides because they are capable of self-associating with hydrogen bonds to form dimeric associations. Acid halides, however, are only capable of receiving hydrogen bonds, not donating them. The structure on acid halides and carboxylic acids that receives hydrogen bonds is the: |
|
A
|
carbon of the carbonyl group |
B
|
oxygen of the carbonyl group |
C
|
oxygen of the hydroxyl group |
D
|
carbon of the R-group |
|
|
|
Tags:
Carboxylic Acids | |
|
| 15 |
Go |
Q:
|
Humins are large, complex organic molecules found in soil that remain insoluble after treatment with dilute alkaline solutions. Which of the following functional groups do humins most likely lack? |
|
A
|
Esters |
B
|
Carboxylic Acids |
C
|
Alcohols |
D
|
Carbonyls |
|
|
|
Tags:
Carboxylic Acids | |
|
| 16 |
Go |
Q:
|
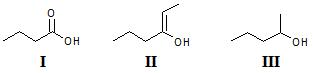
Rank the compounds above in increasing order of acidity. |
|
A
|
I < II < III |
B
|
III < II < I |
C
|
III < I < II |
D
|
I < III < II |
|
|
|
Tags:
Alcohols | Carboxylic Acids | |
|
| 18 |
Go |
Q:
|
A chemical compound is insoluble in water. Upon reaction with NaOH, however, the compound becomes soluble. What has most likely happened to the chemical compound? |
|
A
|
An -O- group or a -COO- group has been protonated |
B
|
An -OH or a -COOH group has been ionized |
C
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbanion |
D
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbocation |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | Carboxylic Acids | |
|
| 19 |
Go |
Q:
|
The relase of CO2 during the Krebs cycle is a result of which type of reaction? |
|
A
|
decarboxylation |
B
|
carbonyl reduction |
C
|
carbonic acid neutralization |
D
|
beta-oxidation |
|
|
|
Tags:
Carboxylic Acids | Citric Acid Cycle | |
|
| 20 |
Go |
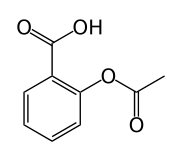
Q:
|
A student wants to perform an acetylation reaction of salicylic acid with acetic anhydride to produce acetylsalisylic acid plus acetic acid. The student begins by mixing the acetic anhydride with water. After adding the salicylic acid, the student notices that no acetylsalisylic acid is produced, but acetic acid is present in the solution. Which of the following best explains the observations? |
|
A
|
The acetic anhydride decomposed in the water into acetic acid. |
B
|
The temperature of the reaction was too low. |
C
|
The student did not add a catalyst to the reaction. |
D
|
The student should have used a heavier anhydride like propanoic anhydride. |
|
|
|
Tags:
Carboxylic Acids | |
|
| 21 |
Go |
Q:
|
Dehydration of two carboxylic acids forms which of the following products? |
|
A
|
ester |
B
|
anhydride |
C
|
ether |
D
|
ketone |
|
|
|
Tags:
Carboxylic Acids | |
|
| 22 |
Go |
Q:
|
A pure carboxylic acid is added to a container with liquid SOCl2 added. The SOCl2 serves which of the following functions? |
|
A
|
acidification of the mixture |
B
|
acts as a desiccant to prevent the acid from absorbing water |
C
|
conversion of the acid to an acid chloride |
D
|
catalyzes the autoreaction of the acid |
|
|
|
Tags:
Acid/Base Equilibria | Carboxylic Acids | |
|
| 23 |
Go |
Q:
|
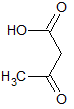
Upon oxidation with excess reagents, which of the following choices best describes the resultant molecule in terms of carboxylic acid and ketone sites present?
 |
|
A
|
One carboxylic acid, two ketones |
B
|
Three ketones |
C
|
Two carboxylic acids |
D
|
One carboxylic acid, one ketone |
|
|
|
Tags:
Carboxylic Acids | Organic Chemistry Reactions | |
|
| 24 |
Go |
Q:
|
Which of the following choices, upon reaction with the molecule below, will NOT result in a carboxylic acid derivative?
 |
|
A
|
SOCl2
|
B
|
PBr3
|
C
|
Cl2
|
D
|
PCl3
|
|
|
|
Tags:
Carboxylic Acids | Acid Derivatives | |
|
| 25 |
Go |
Q:
|
Given the molecule on the left, what will happen to the pKa if an azido group replaces one of the hydrogens from the methyl group? The structure of the azido group is shown to the right of the original molecule
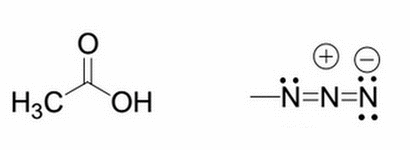 |
|
A
|
Upon addition of the azido group, the molecule's pKa will increase (compared to the original molecule shown on the left without the azido group) because the azido group stabilizes the negatively charged conjugate base
|
B
|
Upon addition of the azido group, the molecule's pKa will decrease (compared to the original molecule shown on the left without the azido group) because the azido group stabilizes the negatively charged conjugate base
|
C
|
Upon addition of the azido group, the molecule's pKa will increase (compared to the original molecule shown on the left without the azido group) because the azido group destabilizes the negatively charged conjugate base
|
D
|
Upon addition of the azido group, the molecule's pKa will decrease (compared to the original molecule shown on the left without the azido group) because the azido group destabilizes the negatively charged conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Carboxylic Acids | |
|
| 26 |
Go |
Q:
|
Carboxyl groups have higher boiling points than acid halides because they are capable of self-associating with hydrogen bonds to form dimeric associations. Acid halides, however, are only capable of receiving hydrogen bonds, not donating them, and cannot self-associate with hydrogen bonds. The structure on acid halides and carboxylic acids that receives hydrogen bonds is the: |
|
A
|
carbon of the carbonyl group |
B
|
oxygen of the carbonyl group |
C
|
oxygen of the hydroxyl group |
D
|
carbon of the R-group |
|
|
|
Tags:
Intermolecular Forces | Carboxylic Acids | Molecular Bonding | |
|
| 27 |
Go |
Q:
|
In the following reaction, the fastest reaction rate occurs in pure water, as opposed to occurring in a solvent like ethanol. Which of the following best describes why this scenario takes place?
 |
|
A
|
The aromatic rings in both reactants are hydrophobic and will be in close proximity to each other, which increases the chance of a reaction taking place
|
B
|
The aromatic rings in both reactants are hydrophobic and will be in close proximity to each other, which decreases the chance of a reaction taking place
|
C
|
The aromatic rings in both reactants are hydrophilic and will be in close proximity to each other, which increases the chance of a reaction taking place
|
D
|
The aromatic rings in both reactants are hydrophilic and will be in close proximity to each other, which decreases the chance of a reaction taking place
|
|
|
|
Tags:
Carboxylic Acids | Molecular Structure | Organic Chemistry Reactions | |
|
| 28 |
Go |
Q:
|
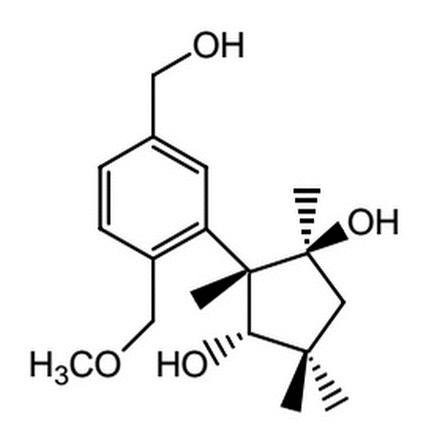
Given the molecule below, which of the following is TRUE regarding its ability (or inability) to undergo an acylation reaction?
 |
|
A
|
The molecule will undergo an intramolecular acylation reaction between the wedged alcohol and the carboxylic acid
|
B
|
The molecule will undergo an intramolecular acylation reaction between the dashed alcohol and the carboxylic acid
|
C
|
The molecule will NOT undergo an intramolecular acylation reaction because the double bond does not allow for the interaction between the alcohols and the carboxylic acid
|
D
|
The molecule will NOT undergo an intramolecular acylation reaction because acylation reactions cannot occur with carboxylic acids.
|
|
|
|
Tags:
Carboxylic Acids | Organic Chemistry Reactions | Molecular Structure | Alcohols | |
|
| 29 |
Go |
Q:
|
Amino acids are characterized by two highly versatile functional groups. Which of the following is NOT a reaction that amino acids can undergo due to these functional groups? |
|
A
|
Nucleophilic addition |
B
|
Esterification |
C
|
Amide bond formation |
D
|
Keto-enol tautomerism |
|
|
|
Tags:
Carboxylic Acids | Protein Structure and Function | Aldehydes and Ketones | |
|
| 30 |
Go |
Q:
|
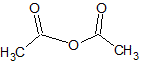
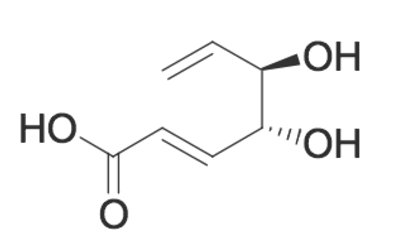
Gallic acid, as shown, is reacted with another carboxylic acid. If water is produced in the reaction, what type of molecule is formed in addition to the water?
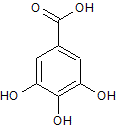
|
|
A
|
acid anhydride |
B
|
ketone |
C
|
aldehyde |
D
|
alcohol |
|
|
|
Tags:
Carboxylic Acids | Acid Derivatives | |
|
| 31 |
Go |
Q:
|
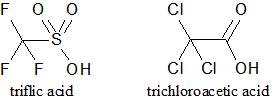
The structures of triflic acid and trichloroacetic acid are shown. How would the pKa of the two acids be compared? |
|
A
|
The pKa of triflic acid would be higher because it is a stronger acid. |
B
|
The pKa of triflic acid would be lower because it is a stronger acid. |
C
|
The pKa of trichloroacetic acid would be higher because it is a stronger acid. |
D
|
The pKa of trichloroacetic acid would be lower because it is a stronger acid. |
|
|
|
Tags:
Acid Derivatives | Carboxylic Acids | Acid/Base Equilibria | |
|
| 33 |
Go |
Q:
|
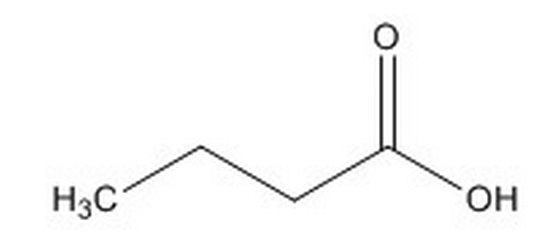
What modification could be made to increase the volatility of a fatty acid? |
|
A
|
Place it a high-pH solution. |
B
|
Convert it to an ester. |
C
|
Lengthen the hydrophobic tail. |
D
|
Decrease the temperature of its solution. |
|
|
|
Tags:
Carboxylic Acids | Acid Derivatives | |
|
| 34 |
Go |
Q:
|
How would a decrease in the pH of a solution affect the volatility of decanoic acid? |
|
A
|
The volatility decreases until the pH equals the pKa at which point the volatility increases. |
B
|
The volatility increases until the pH equals the pKa at which point the volatility decreases. |
C
|
The volatility continues to increase as the pH decreases. |
D
|
The volatility continues to decrease as the pH decreases. |
|
|
|
Tags:
Intermolecular Forces | Carboxylic Acids | |
|
| 36 |
Go |
Q:
|
Which of the following would happen to hexanoic acid in an aqueous solution when the pH his changed from 3 to 6? |
|
A
|
Hexanoic acid would polymerize. |
B
|
Hexanoic acid would become more protonated. |
C
|
Hexanoic acid would become more soluble. |
D
|
Hexanoic acid would precipitate. |
|
|
|
Tags:
Solutions | Carboxylic Acids | Acid/Base Equilibria | |
|
| 37 |
Go |
Q:
|
The carboxylic acid butanoic acid (C3H7COOH) contains how many stereoisomers other than the one shown? |
|
|
|
|
Tags:
Carboxylic Acids | |
|
| 38 |
Go |
Q:
|
The amino acid valine contains which of the following functional groups?
I. amine
II. carboxyl
III. alcohol |
|
A
|
I only |
B
|
I and II only |
C
|
II and III only |
D
|
I, II, and III |
|
|
|
Tags:
Carboxylic Acids | |
|
| 39 |
Go |
Q:
|
Reacting a carboxylic acid with an alcohol in the presence of an acid catalyst would be expected to produce a(n): |
|
A
|
amide. |
B
|
ester. |
C
|
secondary alcohol. |
D
|
ketone. |
|
|
|
Tags:
Carboxylic Acids | Alcohols | |
|
| 40 |
Go |
Q:
|
Which of the following reflects the charge on a glycine molecule at pH of 1? |
|
|
|
|
Tags:
Carboxylic Acids | |
|
| 41 |
Go |
Q:
|
Which functional group is NOT contained in the following molecule?
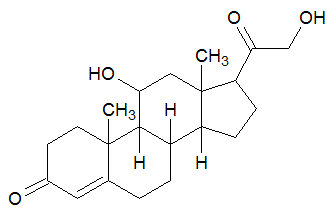 |
|
A
|
carbonyl |
B
|
carboxyl |
C
|
hydroxyl |
D
|
methyl |
|
|
|
Tags:
Carboxylic Acids | |
|
| 42 |
Go |
Q:
|
Which of the following functional groups has the most stable conjugate base? |
|
A
|
alkyne group |
B
|
alkene group |
C
|
carboxylic group |
D
|
alcohol group |
|
|
|
Tags:
Carboxylic Acids | |
|
| 43 |
Go |
Q:
|
Which of the following statements is TRUE of carbonyl compounds? |
|
A
|
A carbonyl compound must include a hydroxyl group. |
B
|
The carbonyl carbon has a partial negative charge. |
C
|
Carbonyl compounds are by definition achiral. |
D
|
The carbonyl carbon is subject to nucleophilic attack. |
|
|
|
Tags:
Carboxylic Acids | Aldehydes and Ketones | |
|
|
We can teach you how to crush the MCAT!
Learn More
|