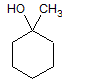|
| 5 |
Go |
Q:
|
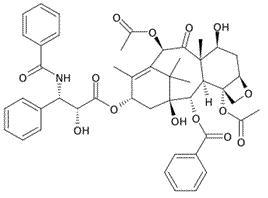
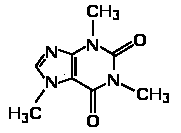
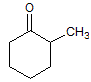
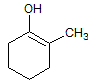
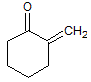
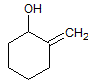
The reaction of a single alcohol with the following compound produces a(n):
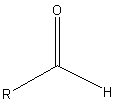 |
|
A
|
acetal |
B
|
hemiacetal |
C
|
ketal |
D
|
hemiketal |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 6 |
Go |
Q:
|
Conversion of a ketone to a secondary alcohol is which type of reaction? |
|
A
|
Oxidation |
B
|
Reduction |
C
|
Alkylation |
D
|
Dehydrogenation |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 7 |
Go |
Q:
|
In the conversion of a ketone to a hemiketal, which of the following changes in the infrared spectrum would indicate completion of the reaction? |
|
A
|
Loss of a strong, sharp peak at 1700 cm-1 and appearance of broad peak around 3200 cm-1 |
B
|
Gain of a strong, sharp peak at 3200 cm-1 and loss of multiple smaller peaks at 2600, 1700, and 1400 cm-1 |
C
|
Gain of a strong, sharp peak at 2200 cm-1 and loss of a strong, sharp peak at 1700 cm-1 |
D
|
A change in the signature of the fingerprint region only |
|
|
|
Tags:
Aldehydes and Ketones | Molecular Structure | |
|
| 8 |
Go |
Q:
|
Alcohols typically have higher boiling points than ketones of similar molecular weight because alcohols |
|
A
|
are more reactive than ketones. |
B
|
are capable of hydrogen bonding. |
C
|
lack symmetry around the carbonyl carbon. |
D
|
lack carbonyl carbon atoms. |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | Intermolecular Forces | |
|
| 9 |
Go |
Q:
|
Which alcohol is more acidic? |
|
A
|
tertiary alcohol |
B
|
secondary alcohol |
C
|
primary alcohol |
D
|
methanol |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 11 |
Go |
Q:
|
Reduction of an aldehyde could produce all of the following EXCEPT: |
|
A
|
carboxylic acid |
B
|
alcohol |
C
|
alkane |
D
|
alkene |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 12 |
Go |
Q:
|
Which of the following reactions can be expected under strongly reducing conditions? |
|
A
|
CH3CHO → CH3COOH |
B
|
CH3CH3 → CH2CH2 |
C
|
CH3COCH3 → CH3CH(OH)CH3 |
D
|
CH3CH2NH2 → CH3CN |
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | |
|
| 13 |
Go |
Q:
|
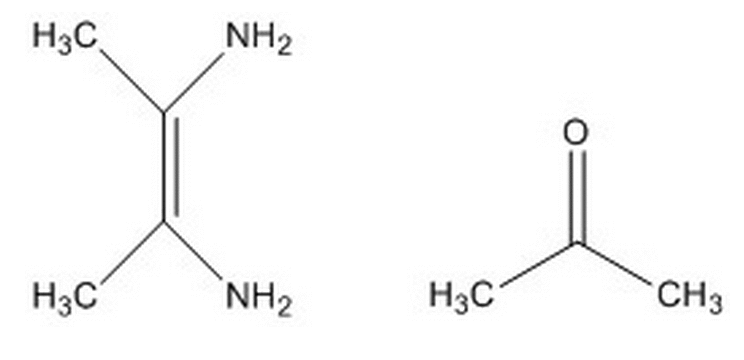
If the molecule below reacts with 2-propanone (the molecule on the right), what is the first step most likely to happen?
 |
|
A
|
Nucleophilic attack by the carbonyl oxygen of 2-propanone on one of the nitrogens
|
B
|
Nucleophilic attack by one of the nitrogens on the carbonyl carbon of 2-propanone
|
C
|
Elimination reaction using the double bond present in the nitrogen-containing molecule
|
D
|
Protonation of 2-propanone in order to create an attractive leaving group
|
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 14 |
Go |
Q:
|
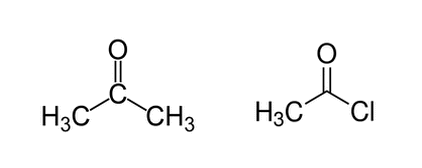
Acetone (on the left) and acetyl chloride (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the lower pKa and why?
 |
|
A
|
The molecule on the left will have the lower pKa and destabilize the conjugate base
|
B
|
The molecule on the left will have the lower pKa and stabilize the conjugate base
|
C
|
The molecule on the right will have the lower pKa and stabilize the conjugate base
|
D
|
The molecule on the right will have the lower pKa and destabilize the conjugate base
|
|
|
|
Tags:
Aldehydes and Ketones | Acid/Base Equilibria | Acid Derivatives | |
|
| 15 |
Go |
Q:
|
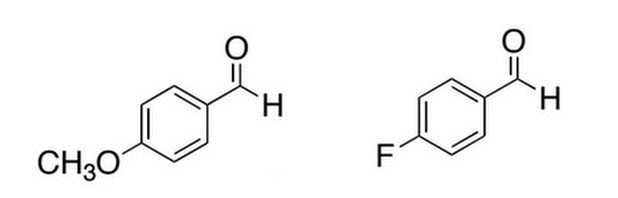
Given the two benzaldehyde derivatives below, which one will react faster (at the carbonyl carbon) with a nucleophile?
 |
|
A
|
The left molecule because the fluoro group is electron-withdrawing, making its carbonyl carbon less electrophilic for addition by a nucleophile
|
B
|
The left molecule because the fluoro group is electron-donating, making its carbonyl carbon less electrophilic for addition by a nucleophile
|
C
|
The right molecule because the fluoro group is electron-withdrawing, making its carbonyl carbon more electrophilic for addition by a nucleophile
|
D
|
The right molecule because the fluoro group is electron-donating, making its carbonyl carbon more electrophilic for addition by a nucleophile
|
|
|
|
Tags:
Chemical Kinetics | Aldehydes and Ketones | Miscellaneous Organic Chemistry | |
|
| 16 |
Go |
Q:
|
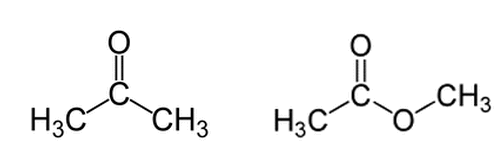
Acetone (on the left) and methyl acetate (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the higher pKa and why?
 |
|
A
|
The molecule on the left will have the higher pKa and destabilize the conjugate base
|
B
|
The molecule on the left will have the higher pKa and stabilize the conjugate base
|
C
|
The molecule on the right will have the higher pKa and destabilize the conjugate base
|
D
|
The molecule on the right will have the higher pKa and stabilize the conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Aldehydes and Ketones | |
|
| 17 |
Go |
Q:
|
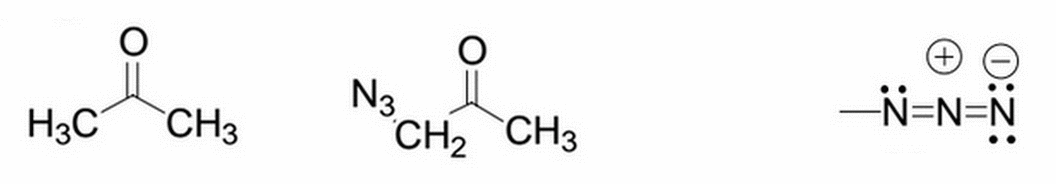
Acetone is shown below on the left. What will happen to the pKa after the addition of an azido group to one of its methyl groups (actually replacing one of the methyl hydrogens)? The site of deprotonation (and thus where the pKa directly refers to) is the hydrogens on the carbon where the azido group was substituted (the -CH2). The modified molecule (after azido addition) is shown in the middle, and the azido group itself is shown on the far right.
 |
|
A
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be lower than the hydrogens on the molecule without the azido (the -CH3) because the azido group stabilizes the conjugate base
|
B
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be higher than the hydrogens on the molecule without the azido (the -CH3) because the azido group stabilizes the conjugate base
|
C
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be lower than the hydrogens on the molecule without the azido (the -CH3) because the azido group destabilizes the conjugate base
|
D
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be higher than the hydrogens on the molecule without the azido (the -CH3) because the azido group destabilizes the conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Aldehydes and Ketones | |
|
| 19 |
Go |
Q:
|
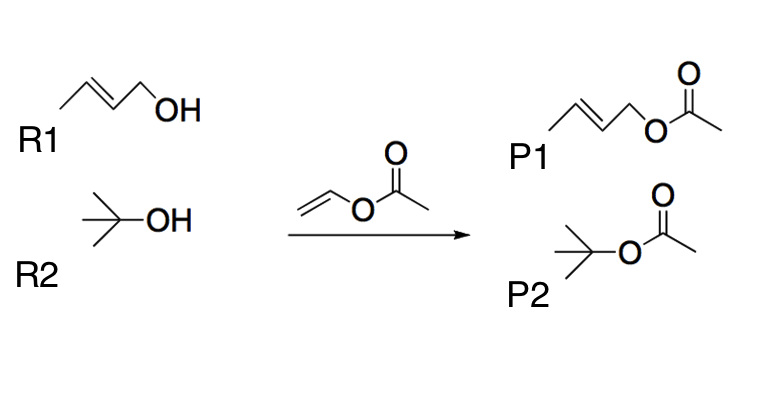
Two different alcohols (R1 and R2) undergo a transesterification reaction using the same reactant, as shown below. Which of the following best explains the most likely product yields?
 |
|
A
|
P1 will have the least yield because R1 is a primary alcohol and will not undergo a transesterification reaction.
|
B
|
P1 will have the most yield because R1 is a primary alcohol and is the least sterically hindered.
|
C
|
P2 will have the most yield because R2 is a tertiary alcohol and is the most sterically hindered.
|
D
|
P2 will have the most yield because R2 is the most basic alcohol.
|
|
|
|
Tags:
Aldehydes and Ketones | Alcohols | Molecular Structure | |
|
| 20 |
Go |
Q:
|
Amino acids are characterized by two highly versatile functional groups. Which of the following is NOT a reaction that amino acids can undergo due to these functional groups? |
|
A
|
Nucleophilic addition |
B
|
Esterification |
C
|
Amide bond formation |
D
|
Keto-enol tautomerism |
|
|
|
Tags:
Carboxylic Acids | Protein Structure and Function | Aldehydes and Ketones | |
|
| 21 |
Go |
Q:
|
An aldehyde reacts with a newly-discovered amine to form an imine molecule. Which of the following reaction types took place in this reaction? |
|
A
|
proton transfer |
B
|
nucleophilic addition |
C
|
acid base reaction |
D
|
rearrangement reaction |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 22 |
Go |
Q:
|
Which of the following holds true for carbonyl groups? |
|
A
|
there is a partial positive charge on the oxygen atom. |
B
|
there is a partial negative charge on the carbon atom. |
C
|
there is a triple bond between the carbon and oxygen atoms. |
D
|
the stereochemistry of the carbonyl group is of a planar geometry. |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 23 |
Go |
Q:
|
The aldehyde propionaldehyde will react with LiAlH4 to form: |
|
A
|
an alcohol via reduction. |
B
|
an alcohol via oxidation. |
C
|
a carboxylic acid via reduction. |
D
|
a carboxylic acid via oxidation. |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 24 |
Go |
Q:
|
Diabetic ketoacidosis is a condition in which individuals with diabetes who have a sudden shortage of insulin, which may result in ketogenesis. Which of the following molecules would be expected to be seen in higher concentrations in the blood in an individual with diabetic ketoacidosis. |
|
A
|
acetone |
B
|
linoleic acid |
C
|
insulin |
D
|
acetic acid |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 25 |
Go |
Q:
|
Molecules of the general formula R2C(OH)CN are called: |
|
A
|
cyanohydrins. |
B
|
amides. |
C
|
imides. |
D
|
amino acids. |
|
|
|
Tags:
Aldehydes and Ketones | |
|
| 26 |
Go |
Q:
|
Which of the following holds true regarding tautomerization? |
|
A
|
tautomers are molecules which differ in one or more stereocenters |
B
|
tautomers are defined as molecules differing in the placement of two or more atoms within a molecule |
C
|
equilibrium between tautomers rarely favors a single tautomer |
D
|
generally the keto form is more stable than the enol form in keto-enol tautomerization |
|
|
|
Tags:
Aldehydes and Ketones | Organic Chemistry Reactions | |
|
| 27 |
Go |
Q:
|
Which of the following statements is TRUE of carbonyl compounds? |
|
A
|
A carbonyl compound must include a hydroxyl group. |
B
|
The carbonyl carbon has a partial negative charge. |
C
|
Carbonyl compounds are by definition achiral. |
D
|
The carbonyl carbon is subject to nucleophilic attack. |
|
|
|
Tags:
Carboxylic Acids | Aldehydes and Ketones | |
|
|
We can teach you how to crush the MCAT!
Learn More
|