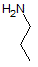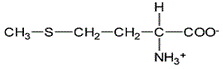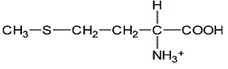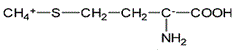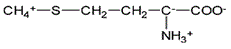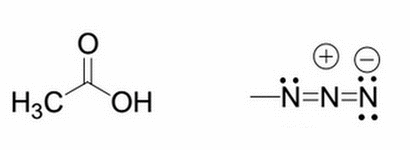|
| 1 |
Go |
Q:
|
Methanethiol, CH3SH, has a pKa of 10.3 and methanol, CH3OH, has a pKa of 15.5. Which is a stronger acid? Which is a stronger base, CH3S- or CH3O-? |
|
A
|
Methanethiol is the stronger acid, its conjugate base is the stronger base |
B
|
Methanol is the stronger acid, its conjugate base is the stronger base |
C
|
Methanethiol is the stronger acid, methanol's conjugate base is the stronger base |
D
|
Methanol is the stronger acid, methanethiol's conjugate base is the stronger base |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 2 |
Go |
Q:
|
Rank the following compounds in order of the most acidic to the least acidic.
I. p-chlorobenzoic acid
II. benzoic acid
III. p-methylbenzoic acid
IV. p-nitrobenzoic acid
|
|
A
|
I > II > III > IV |
B
|
IV > I > II > III |
C
|
IV > II > I > III |
D
|
III > IV > II > I |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 3 |
Go |
Q:
|
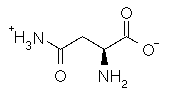
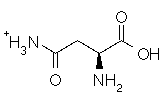
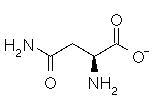
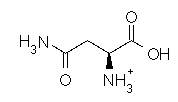
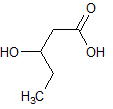
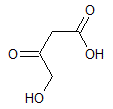
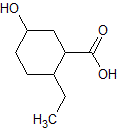
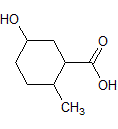
Asparagine has a pI of 5.41. Which of the following pictures depicts asparagine at a pH of 2? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 4 |
Go |
Q:
|
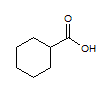
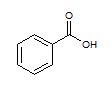
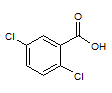
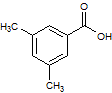
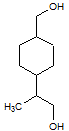
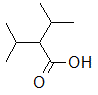
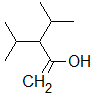
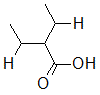
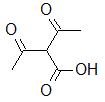
Rank the following molecules in descending order of acidity (most acidic first).
I. 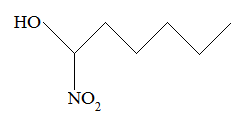
II. 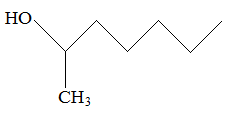
III. 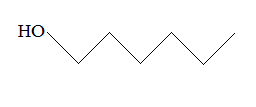 |
|
A
|
I, II, III |
B
|
I, III, II |
C
|
III, I, II |
D
|
III, II, I |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 5 |
Go |
Q:
|
Knowing that the Ka of triflic acid (monoprotic) is 8.0 x 1014 and will almost completely dissociate in solution, what is the pH of a 1M solution of triflic acid?
|
|
A
|
8.0 x 1014 |
B
|
1/(8.0 x 1014) |
C
|
0 |
D
|
1 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 6 |
Go |
Q:
|
CO2 dissolves in water. Once dissolved, |
|
A
|
it decreases the pH of the solution due to formation of HCO2 + OH- |
B
|
it decreases the pH of the solution due to formation of HCO3 + H+ |
C
|
it increases the pH of the solution due to formation of HCO2 + OH- |
D
|
it increases the pH of the solution due to formation of HCO3 + H+ |
|
|
|
Tags:
Acid/Base Equilibria | Solutions | |
|
| 7 |
Go |
Q:
|
Given that the pOH of a particular solution is 0.5, the pH can be calculated to be |
|
A
|
0.5 |
B
|
11.5 |
C
|
13.5 |
D
|
impossible to determine from the information given |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 8 |
Go |
Q:
|
A solution has a hydronium ion concentration of 0.001 M. What is the pOH of the solution? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 9 |
Go |
Q:
|
What is the [H+] concentration of a solution of pH 4? |
|
A
|
1.0 x 10-3 |
B
|
1.0 x 10-4 |
C
|
1.0 x 10-5 |
D
|
1.0 x 10-14 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 10 |
Go |
Q:
|
Oxalic acid, H2C2O4, is a diprotic acid with a pKa value of 1.19. If 0.10 M oxalic acid is titrated 0.10 M NaOH, what would be the pH halfway to the first equivalence point? Relevant equilibria are given below:
H2C2O4 + H2O ↔ H3O+ + HC2O4- (pKa1 = 1.19)
HC2O4- + H2O ↔ H3O+ + C2O4-2 (pKa2 = 4.21) |
|
A
|
1.19 |
B
|
4.21 |
C
|
2.70 |
D
|
2.11 |
|
|
|
Tags:
Acid/Base Equilibria | Titrations | |
|
| 12 |
Go |
Q:
|
The Ka value of a weak acid, HA, is 10-6. An initial solution containing 1.0 M of HA is prepared. What is the pH of the final solution at equilibrium? |
|
A
|
pH 3 |
B
|
pH 4 |
C
|
pH 5 |
D
|
pH 6 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 13 |
Go |
Q:
|
The concentration of hydroxide in an aqueous solution is 1x10-3 M. What is the pH of the solution? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 15 |
Go |
Q:
|
What volume of a 0.4 M solution of NaOH is necessary to take the pH of a 36 ml solution of 0.8 M HCl to 7.0? |
|
A
|
18 mL |
B
|
32 mL |
C
|
36 mL |
D
|
72 mL |
|
|
|
Tags:
Acid/Base Equilibria | Titrations | |
|
| 16 |
Go |
Q:
|
Which of the following is the most basic? |
|
A
|
(CH3)3COH |
B
|
(CH3)2CHOH |
C
|
(CH3)CH2OH |
D
|
CH3OH |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 17 |
Go |
Q:
|
Arrange the following oxyacids in descending order of acid strength. |
|
A
|
perchloric acid > chlorous acid > chloric acid > hypochlorous acid |
B
|
perchloric acid > chloric acid > chlorous acid > hypochlorous acid |
C
|
hypochlorous acid > chloric acid > chlorous acid > perchloric acid |
D
|
hypochlorous acid > chlorous acid > chloric acid > perchloric acid |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 18 |
Go |
Q:
|
Which of the following is true of the difference between pH and pKa? |
|
A
|
pH is on a log scale whereas pKa is not. |
B
|
pH can only go from a scale of 0 to 14 whereas pKa is not subject to the same restrictions. |
C
|
pH and pKa are identical concepts |
D
|
pH depends on the amount of acid in solution whereas pKa does not. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 19 |
Go |
Q:
|
Where the pH is equal to the pKa for a monoprotic acid, |
|
A
|
the acid is completely protonated |
B
|
half of the acid has been deprotonated |
C
|
all of the acid has been deprotonated |
D
|
the level of deprotonation depends upon whether the pKa is greater than or less than 7 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 20 |
Go |
Q:
|
During a purification experiment, Compound X is extracted from ethyl acetate in which it is readily soluble into an aqueous Solution A in which it is only moderately soluble. Compound X has a pKa of 2.8 and has a carboxylic acid as its primary functional group. In the next step, the student wants to precipitate Compound X and attempts different experimental conditions. The observations from two conditions are shown in the table below.
| � |
Condition 1 |
Condition 2 |
| pH |
6.5 |
0.5 |
Assuming the there is no difference in the experimental conditions except for the pH, which of the following is true? |
|
A
|
Condition 1 will precipitate Compound X because Compound X will be insoluble when the pH is above the pKa |
B
|
Condition 2 will precipitate Compound X because Compound X will be insoluble when the pH is below the pKa |
C
|
Condition 1 will precipitate Compound X because Compound X will be insoluble when the pH is below the pKa |
D
|
Condition 2 will precipitate Compound X because Compound X will be insoluble when the pH is above the pKa |
|
|
|
Tags:
Acid/Base Equilibria | Solutions | Carboxylic Acids | |
|
| 21 |
Go |
Q:
|
Which of the following does not hold true for a glass of pure water at 40 °C? |
|
A
|
[H+] = [OH-] |
B
|
The expression for the equilibrium constant for the dissociation of water does not include the term for liquid water |
C
|
The solution will contain a nonzero concentration of dissociated hydroxide ions |
D
|
pH is 7 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 22 |
Go |
Q:
|
A 12.5mL sample of 3.0M malic acid (a strong, diprotic acid) was added to 30mL sodium hydroxide. If the malic acid becomes completely deprotonated, what is the molarity of the sodium hydroxide that was added? |
|
A
|
1 M |
B
|
2 M |
C
|
1.25 M |
D
|
2.5 M |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 23 |
Go |
Q:
|
Which of the following values of Ka correspond to the strongest acid? |
|
A
|
5.9 x 10-7 |
B
|
3.3 x 10-2 |
C
|
7.1 x 105 |
D
|
1.8 x 109 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 24 |
Go |
Q:
|
A newly-discovered weak acid has a pKa of 4.8. A solution of this acid is found to have a pH of around 4.8. The concentration of the protonated form is increased 1000-fold. Which of the following is closest to the new pH of the solution? |
|
|
|
|
Tags:
Acid/Base Equilibria | Quantitative Skills | |
|
| 25 |
Go |
Q:
|
Which of the following compounds would be expected to have the strongest conjugate base? |
|
A
|
methanol |
B
|
ethanol |
C
|
1-propanol |
D
|
1-butanol |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 26 |
Go |
Q:
|
Which of the following may hold true of a compound if an increase in pH of the solvent results in increased solubility of the compound? |
|
A
|
The compound is an acid |
B
|
The compound is a base |
C
|
The compound contains an ionic bond |
D
|
The compound has charge at neutral pH |
|
|
|
Tags:
Acid/Base Equilibria | Solutions | |
|
| 27 |
Go |
Q:
|
The Ka for acetic acid is 1.8 x 10-5. In a solution of 35% acetic acid, which of the following species would be most abundant? |
|
A
|
HAc |
B
|
Ac- |
C
|
H+ |
D
|
The answer cannot be determined with the given information |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 28 |
Go |
Q:
|
Which of the following acids will have the lowest pH as a 1 M aqueous solution? |
|
A
|
HF; Ka = 6.6 x 10-4 |
B
|
HCl; Ka = 1.3 x 106 |
C
|
HBr; Ka = 1 x 109 |
D
|
HI; Ka = 3.2 x 109 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 29 |
Go |
Q:
|
The pKa of aniline, or C6H5NH2 is 4.60 while the pKa of benzylamine, or C6H5CH2NH2 is 9.34. Which of the following best explains the disparity in their pKa values? |
|
A
|
The larger size of benzylamine increases its pKa. |
B
|
The CH2 group has electron-donating properties and separates the amine from the electron-withdrawing benzene ring. |
C
|
The CH2 group adds electron-withdrawing characteristics to raise the pKa. |
D
|
The difference in steric hindrance between the two is sufficient to explain the difference in pKa. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 30 |
Go |
Q:
|
The inductive effect can stabilize the strength of an organic acid. Which of the following structures would have the strongest inductive effect, and therefore have the strongest acidic group? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 31 |
Go |
Q:
|
What would be the expected pKa value for the amino group on the amino acid glycine? |
|
A
|
Between 0 and 2 |
B
|
Between 2 and 7 |
C
|
Between 7 and 10 |
D
|
Between 10 and 12 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 32 |
Go |
Q:
|
pH indicators are usually organic molecules whose colors change when the pH is in the range of the indicator pKa. This is best explained by which of the following? |
|
A
|
The protonation/deprotonation events occur at the half-equivalence point and change the structure and color of the indicator molecule. |
B
|
The indicator is least stable at its half-equivalence point, causing it to change color very easily. |
C
|
Titrations of pH indicators, being exothermic reactions, reach critical temperatures at the half-equivalence point. |
D
|
There is no direct correlation between the pH and color of a pH indicator. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 35 |
Go |
Q:
|
Which of the following is the strongest acid? |
|
A
|
HI (Ka = 3.2 x 109) |
B
|
H2SO4 (Ka = 1.0 x 103) |
C
|
HNO3 (Ka = 2.4 x 101) |
D
|
HCl (Ka = 1.3 x 106) |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 36 |
Go |
Q:
|
A 0.01 M solution of HCl would have a pH of approximately 2. For HF, which has a Ka lower than that of HCl, a 0.01 M solution would have a pH that would be: |
|
A
|
less than 2. |
B
|
equal to 2. |
C
|
greater than 2. |
D
|
The answer cannot be determined from the given information. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 37 |
Go |
Q:
|
For the pH of a solution to be estimated accurately using only the concentration of the acid present, which of the following must be true? |
|
A
|
The Ka of the acid is very high. |
B
|
The concentration of H+ must equal the concentration of the conjugate base A-. |
C
|
The acid must be a weak acid; the pH of strong acids cannot be calculated directly in this way. |
D
|
The acid must be concentrated; the estimation does not work at high dilution. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 38 |
Go |
Q:
|
A particular solution of a concentrated acid has a pH of 2. The water is evaporated until half the volume remains, leaving the acid twice as concentrated. What has happened to the pH of the solution? |
|
A
|
The pH is equal to or below 1. |
B
|
The pH is between 1 and 2. |
C
|
The pH remains at 2. |
D
|
The pH is between 2 and 4. |
|
|
|
Tags:
Acid/Base Equilibria | Solutions | |
|
| 39 |
Go |
Q:
|
A buffer solution can be prepared with a weak acid combined with which of the following? |
|
A
|
a weak base |
B
|
water |
C
|
the weak acid's conjugate base |
D
|
a strong acid |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 42 |
Go |
Q:
|
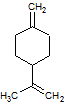
When the compound above undergoes a hydration reaction after treatment with aqueous H2SO4, which of the following compounds will be the major product? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 43 |
Go |
Q:
|
What is the conjugate base of the bicarbonate ion HCO3-? |
|
A
|
H2CO3 |
B
|
CO32- |
C
|
H2O |
D
|
OH- |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 44 |
Go |
Q:
|
Which of the following substances is NOT an acid? |
|
A
|
H3O+ |
B
|
NH4+ |
C
|
HSO4- |
D
|
CH3NH2 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 45 |
Go |
Q:
|
Which of the following substances can act as both an acid and a base? |
|
A
|
H2O |
B
|
NH4+ |
C
|
O2- |
D
|
SO42- |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 47 |
Go |
Q:
|
Which of the following compounds is an electrolyte at neutral pH? |
|
A
|
CH3COOH |
B
|
CH3CH2OH |
C
|
H2CO |
D
|
CH3C(OH)3 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 48 |
Go |
Q:
|
The compound below would most easily become soluble in water when treated with a dilute solution of which of the following?
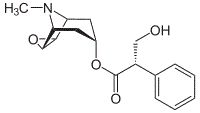 |
|
A
|
HCl |
B
|
NaOH |
C
|
NH4OH |
D
|
KBr |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 49 |
Go |
Q:
|
A chemical compound is insoluble in water. Upon reaction with NaOH, however, the compound becomes soluble. What has most likely happened to the chemical compound? |
|
A
|
An -O- group or a -COO- group has been protonated |
B
|
An -OH or a -COOH group has been ionized |
C
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbanion |
D
|
A hydrogen from an alkyl group has been lost to OH- to make H2O and a water soluble carbocation |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | Carboxylic Acids | |
|
| 52 |
Go |
Q:
|
Replacing an alkyl group with an electron-withdrawing group on an organic acid would |
|
A
|
increase the strength of the acid |
B
|
decrease the stability of its conjugate base |
C
|
increase the pKa of the acid |
D
|
decrease the Ka of the acid |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 53 |
Go |
Q:
|
Ca(OH)2 has Ksp = 10-6. If 0.1 mol of Ca(OH)2 was added to 1 L of pure water, what would be the approximate pH of the resulting solution? |
|
|
|
|
Tags:
Acid/Base Equilibria | Solutions | |
|
| 54 |
Go |
Q:
|
In the diagram below, given the pKa of -OH1 is 8.24 and the pKa of -OH2 is 7.81, determine which of the structures will be most abundant in solution at a pH of 8.00.
 |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 55 |
Go |
Q:
|
Eosin and hematoxylin are two compounds used in staining cellular components for examination under the microscope. Eosin stains positively-charged molecules (e.g. the cytosol) and hematoxylin stains negatively-charged molecules (e.g. the nucleus). Hematoxylin provides a dark purple coloration whereas eosin provides a pinkish hue. If the cytosol of the cell drops in pH, what would we expect to occur to the coloration of the cytosol? |
|
A
|
The color will remain the same |
B
|
The cytosol will have a stronger pink stain |
C
|
The cytosol will have a weaker pink stain |
D
|
Not enough information provided |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 56 |
Go |
Q:
|
Which substance can be classified as an Arrhenius acid? |
|
A
|
HCl |
B
|
NaCl |
C
|
LiOH |
D
|
KOH |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 57 |
Go |
Q:
|
At a pH of 13, what is the expected difference in charge between the amino acids glutamic acid (Glu) and methionine (Met)?
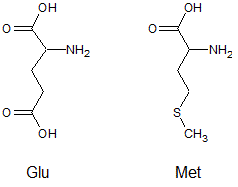 |
|
A
|
Glu and Met have the same charge |
B
|
Glu has a charge 1 unit greater than Met |
C
|
Glu has a charge 2 units less than Met |
D
|
Glu has a charge 1 unit less than Met |
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 58 |
Go |
Q:
|
Nitrate has a pKa that is less than zero. In order for nitrate to form a buffer solution, it must be prepared with: |
|
A
|
water. |
B
|
nitrite. |
C
|
its conjugate base. |
D
|
its conjugate acid. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 59 |
Go |
Q:
|
Reduction potential is most closely related to which of the following? |
|
A
|
electron affinity |
B
|
electronegativity |
C
|
ionization energy |
D
|
oxidation state |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 60 |
Go |
Q:
|
Reduction potential is most closely related to which of the following? |
|
A
|
electron affinity |
B
|
electronegativity |
C
|
ionization energy |
D
|
oxidation state |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 61 |
Go |
Q:
|
A modification on a chemical compound results in the shifting of its pKa value from 2.0 to 4.0. Compared to the half-equivalence point for the original compound, the new compound will reach its half equivalence point when the hydronium ion concentration is: |
|
A
|
4 times less than the original compound |
B
|
2 times less than the original compound |
C
|
100 times less than for the original compound |
D
|
10 times less than the original compound |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 62 |
Go |
Q:
|
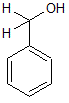
Which of the following would increase the acidity of the hydroxyl group in the compound shown above? |
|
A
|
Addition of methyl groups to the benzene ring |
B
|
Replacement of the hydrogen atoms in -CH2OH with two methyl groups |
C
|
Adding HCl to the aqueous solution of the compound |
D
|
Addition of chlorine atoms to the benzene ring |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 63 |
Go |
Q:
|
Proteins distributed on a pH gradient and separated by their isoelectric point will be located at which position? |
|
A
|
The point where the protein has denatured and is linear. |
B
|
The point where the protein's size corresponds to the pore size of the gel matrix. |
C
|
The point where the protein has been completely deprotonated. |
D
|
The point where the protein's pKa is equal to the external pH. |
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 64 |
Go |
Q:
|
Which of the following compounds would be expected to be the strongest base? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 66 |
Go |
Q:
|
NH4OH is considered to be a base because: |
|
A
|
The nitrogen accepts protons from solution |
B
|
The -OH group accepts protons from solution |
C
|
it donates OH- to solution |
D
|
The nitrogen donates electrons to solution |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 67 |
Go |
Q:
|
Glucose oxidase catalyses the conversion of glucose into gluconic acid with a pKa of 3.9. The buffer for glucose oxidase should be at pH 7 to maximize the activity of the enzyme. The gluconic acid produced would predominantly be in which state? |
|
A
|
protonated |
B
|
deprotonated |
C
|
the protonated and deprotonated states would be roughly equivalent in concentration |
D
|
there is no relationship between the protonation and the pH of the buffer |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 68 |
Go |
Q:
|
Which of the following would be expected to be the strongest acid? |
|
A
|
CH2FCOOH |
B
|
CH2ClCOOH |
C
|
CH2BrCOOH |
D
|
CH3COOH |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 69 |
Go |
Q:
|
An unknown acid is found to dissociate in a 1:108 ratio (dissociates almost entirely) when dissolved in water. Its Ka can be approximated to be: |
|
A
|
near 0. |
B
|
much less than 1. |
C
|
approximately equal to 1. |
D
|
much greater than 1. |
|
|
|
Tags:
Acid/Base Equilibria | Quantitative Skills | |
|
| 70 |
Go |
Q:
|
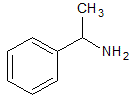
The solubility in water of the compound above increases dramatically when which of the following occurs? |
|
A
|
The methyl group is replaced with an ethyl group |
B
|
The amine is methylated |
C
|
The C-CH3 group is replaced with C=O to make an amide |
D
|
The pH of the solution is lowered below the pKa of the compound |
|
|
|
Tags:
Acid/Base Equilibria | Intermolecular Forces | |
|
| 71 |
Go |
Q:
|
Dissolved CO2 lowers the pH of a solution because: |
|
A
|
CO2 can act as a Lewis acid and accept electrons |
B
|
CO2 causes the dissociation of water and reacts with OH-, leaving behind H+ |
C
|
CO2 reacts with water to form carbonic acid, H2CO3 |
D
|
CO2 prevents the dissociation of water and decreases the amount of OH- released to solution |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 72 |
Go |
Q:
|
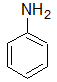
Aniline
Aniline is a considerably weaker base than many aliphatic amines of comparable molecular weight because: |
|
A
|
aniline exhibits much less steric hindrance than an aliphatic group of comparable size. |
B
|
amines must be completely substituted to act as strong bases. |
C
|
the resonance stabilization of the benzene ring allows it to act as an electron-withdrawing group, inhibiting the basicity of the amine. |
D
|
the benzene ring hydrogen bonds with the amine, hindering its ability to act as a base. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 74 |
Go |
Q:
|
In the reaction between BH3 and N(CH3)3 to form BH3N(CH3)3, the N(CH3)3 acts as which of the following? |
|
A
|
Lewis acid |
B
|
Lewis base |
C
|
Bronsted-Lowry acid |
D
|
Bronsted-Lowry base |
|
|
|
Tags:
Acid/Base Equilibria | Molecular Bonding | |
|
| 75 |
Go |
Q:
|
Which of the following species would be LEAST abundant in a solution of H3PO4 at very low pH? |
|
A
|
H3PO4 |
B
|
H2PO4- |
C
|
PO43- |
D
|
H3O+ |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 76 |
Go |
Q:
|
Soluble proteins like blood serum albumin can help buffer pH levels because: |
|
A
|
the acidic and basic amino acid residues can accept or donate protons in solution as needed. |
B
|
soluble proteins can precipitate when the pH is altered. |
C
|
proteins can hydrolize in the presence of acids or bases which neutralizes their effect. |
D
|
proteins can activate other acid- or base-producing proteins to alter the pH. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 77 |
Go |
Q:
|
Enols are generally stronger acids than alkyl alcohols of similar structure because: |
|
A
|
enols have a lower molecular weight |
B
|
enols have more electron-donating characteristic |
C
|
enols can resonance stabilize the deprotonated form |
D
|
enols are more reactive than alkyl alcohols |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 78 |
Go |
Q:
|
A pure carboxylic acid is added to a container with liquid SOCl2 added. The SOCl2 serves which of the following functions? |
|
A
|
acidification of the mixture |
B
|
acts as a desiccant to prevent the acid from absorbing water |
C
|
conversion of the acid to an acid chloride |
D
|
catalyzes the autoreaction of the acid |
|
|
|
Tags:
Acid/Base Equilibria | Carboxylic Acids | |
|
| 79 |
Go |
Q:
|
What is the conjugate acid of the ion HPO42-? |
|
A
|
PO43- |
B
|
H2PO4- |
C
|
H+ |
D
|
H3SO4 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 80 |
Go |
Q:
|
Which of the following is expected to have the highest pKa? |
|
A
|
hydrofluoric acid |
B
|
hydrochloric acid |
C
|
hydrobromic acid |
D
|
hydroiodic acid |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 81 |
Go |
Q:
|
Weak bases, when titrated with strong acids, have an equivalent point which deviates from 7 (pH neutral). The reason for this phenomena is: |
|
A
|
at the equivalence point, there will be fewer hydrogen ions than expected. |
B
|
at the equivalence point, there will be no protons in solution. |
C
|
at the equivalence point, the base will have a lower concentration than expected. |
D
|
at the equivalence point, the conjugate acid will exist in a lower concentration than expected. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 82 |
Go |
Q:
|
For the compound XCH2CH2COOH, which of the following, if substituted for X, would result in the strongest acid? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 83 |
Go |
Q:
|
Which of the following mixtures (if added in a 1:1 ratio) will have the highest pH? |
|
A
|
Strong acid, weak base |
B
|
Strong acid, strong base |
C
|
Weak acid, strong base |
D
|
Weak acid, weak base |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 84 |
Go |
Q:
|
The conjugate base of hydrogen phosphate (HPO42-) is: |
|
A
|
OH-. |
B
|
PO43-. |
C
|
H2PO4-. |
D
|
H3PO4. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 85 |
Go |
Q:
|
Which of the following is the sum of the concentrations of hydrogen ions and hydroxide ions in neutral water? |
|
A
|
10-14 |
B
|
10-7 |
C
|
7.0 |
D
|
2x10-7 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 86 |
Go |
Q:
|
A weak acid (HA) is titrated in a beaker with a strong base (B-) such that equivalent molar amounts of each is added. Which of the following substances will not be found in the beaker at the end of titration?
(I) HA
(II) A-
(III) B-
(IV) HB |
|
A
|
All of the above substances will be present in solution |
B
|
I and III only |
C
|
III only |
D
|
I, II, and III only |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 88 |
Go |
Q:
|
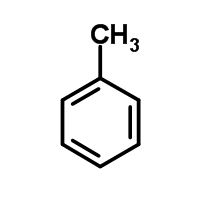
The molecule below has a pKa greater than 40. If a neutral nitrogen replaces the carbon at the fourth position on the ring, what will happen to the pKa and why?
 |
|
A
|
The pKa will increase because the introduction of the nitrogen atom eliminates any resonance
|
B
|
The pKa will increase because once the molecule is deprotonated, the negative charge can be stabilized by the more electronegative nitrogen atom
|
C
|
The pKa will decrease because the introduction of the nitrogen atom eliminates any resonance
|
D
|
The pKa will decrease because once the molecule is deprotonated, the negative charge can be stabilized by the more electronegative nitrogen atom
|
|
|
|
Tags:
Acid/Base Equilibria | Molecular Structure | |
|
| 89 |
Go |
Q:
|
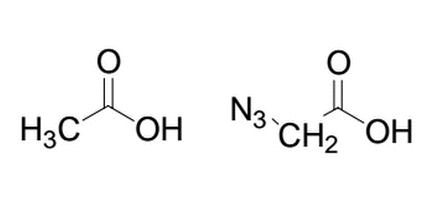
Given the two similar molecules below, which will have the lower pka after deprotonation of the alcohol group and why?
 |
|
A
|
The left molecule because the carbon stabilizes the conjugate base better than the nitrogen group |
B
|
The left molecule because the nitrogen group is electron-withdrawing, which stabilizes the negatively charged conjugate base |
C
|
The right molecule because the nitrogen group will accept a proton, stabilizing the conjugate base |
D
|
The right molecule because the nitrogen group is electron-withdrawing, which stabilizes the negatively charged conjugate base |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 90 |
Go |
Q:
|
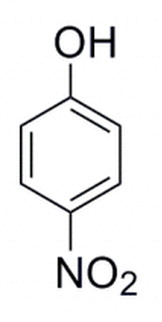
The molecule below is developed in the laboratory. A separate molecule is constructed where the NO2 group moves to the adjacent carbon atom. This alcohol is then deprotonated. The pka would be expected to:
 |
|
A
|
Increase because the modification allows for better resonance to stabilize the negative charge on the conjugate base |
B
|
Decrease because the modification allows for better resonance to stabilize the negative charge on the conjugate base |
C
|
Increase because the modification eliminates the resonance stabilization of the negative charge on the conjugate base |
D
|
Decrease because the modification eliminates the resonance stabilization of the negative charge on the conjugate base |
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 91 |
Go |
Q:
|
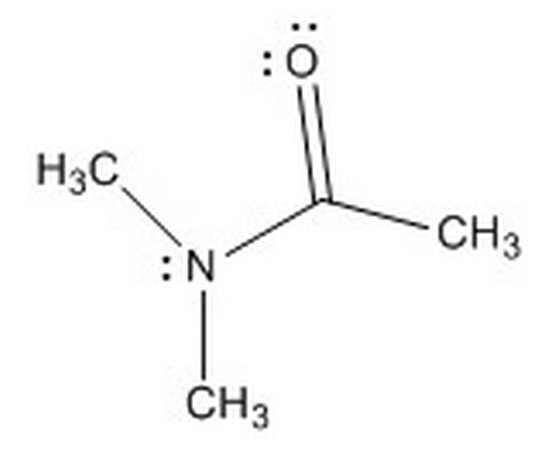
Given the molecule below, and the choice between the nitrogen and oxygen atoms, where will protonation more likely occur and why?
 |
|
A
|
Oxygen because there are no delocalized electrons present, meaning it is a stronger base when protonated
|
B
|
Nitrogen because the delocalized electrons on the nitrogen make it more stable, and thus, a stronger base than the oxygen
|
C
|
Oxygen because the delocalized electrons on the nitrogen make it more stable, and thus, a weaker base than the oxygen
|
D
|
Nitrogen because it is more electronegative than oxygen, and once protonated, will have a lower pka than the protonated oxygen
|
|
|
|
Tags:
Acid/Base Equilibria | Molecular Structure | |
|
| 92 |
Go |
Q:
|
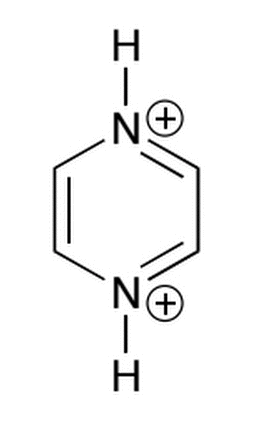
The molecule below has two sites for deprotonation, with corresponding pka values of approximately -6 and 0. Which of the following correctly describes the state of this molecule when dissolved in pH buffers of 10 and 5?
 |
|
A
|
For both buffers, both nitrogen sites will remain protonated
|
B
|
For both buffers, both nitrogen sites will be deprotonated
|
C
|
For both buffers, the nitrogen site with the lower pka will be deprotonated, and the other will not
|
D
|
For both buffers, the nitrogen site with the higher pka will be deprotonated, and the other will not
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 93 |
Go |
Q:
|
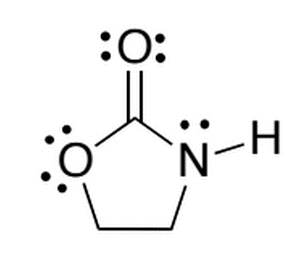
The molecule below is synthesized in a lab. If the four hydrogens (currently bonded to the two sp3 carbons) were replaced with four fluorines, which of the following best describes the acidity of the new molecule compared to the original?
 |
|
A
|
More acidic, since deprotonation would result in a better stabilized conjugate base due to the electronegative fluorines providing an inductive effect
|
B
|
Less acidic, since the fluorine atoms provide resonance to the nitrogen atom, allowing for easier deprotonation of its hydrogen
|
C
|
More acidic, since the fluorine atoms provide resonance to the nitrogen atom, allowing for easier deprotonation of its hydrogen
|
D
|
Less acidic, since deprotonation would result in a better stabilized conjugate base due to the electronegative fluorines providing an inductive effect
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 94 |
Go |
Q:
|
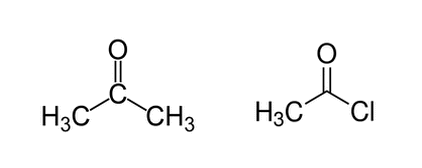
Acetone (on the left) and acetyl chloride (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the lower pKa and why?
 |
|
A
|
The molecule on the left will have the lower pKa and destabilize the conjugate base
|
B
|
The molecule on the left will have the lower pKa and stabilize the conjugate base
|
C
|
The molecule on the right will have the lower pKa and stabilize the conjugate base
|
D
|
The molecule on the right will have the lower pKa and destabilize the conjugate base
|
|
|
|
Tags:
Aldehydes and Ketones | Acid/Base Equilibria | Acid Derivatives | |
|
| 95 |
Go |
Q:
|
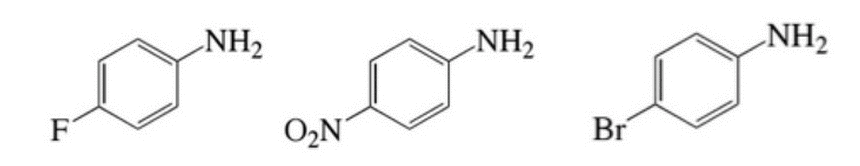
With regard to the anticipated basicity of the NH2 on each of the ring systems below, which of the following correctly orders the 3 molecules from most basic to least basic? Key: A = left molecule, B = middle molecule, C = right molecule
 |
|
A
|
C, A, B
|
B
|
B, A, C
|
C
|
C, B, A
|
D
|
B, C, A
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 96 |
Go |
Q:
|
Acetone (on the left) and methyl acetate (on the right) are shown below. Regarding deprotonation of a hydrogen from the left methyl group (for both molecules), which molecule will have the higher pKa and why?
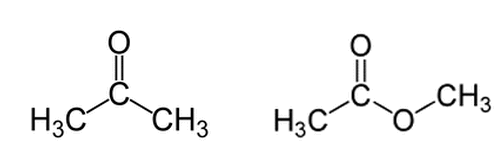 |
|
A
|
The molecule on the left will have the higher pKa and destabilize the conjugate base
|
B
|
The molecule on the left will have the higher pKa and stabilize the conjugate base
|
C
|
The molecule on the right will have the higher pKa and destabilize the conjugate base
|
D
|
The molecule on the right will have the higher pKa and stabilize the conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Aldehydes and Ketones | |
|
| 97 |
Go |
Q:
|
Given the molecule on the left, what will happen to the pKa if an azido group replaces one of the hydrogens from the methyl group? The structure of the azido group is shown to the right of the original molecule
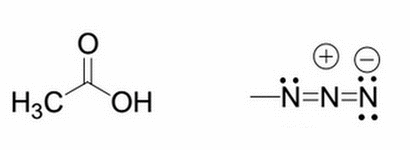 |
|
A
|
Upon addition of the azido group, the molecule's pKa will increase (compared to the original molecule shown on the left without the azido group) because the azido group stabilizes the negatively charged conjugate base
|
B
|
Upon addition of the azido group, the molecule's pKa will decrease (compared to the original molecule shown on the left without the azido group) because the azido group stabilizes the negatively charged conjugate base
|
C
|
Upon addition of the azido group, the molecule's pKa will increase (compared to the original molecule shown on the left without the azido group) because the azido group destabilizes the negatively charged conjugate base
|
D
|
Upon addition of the azido group, the molecule's pKa will decrease (compared to the original molecule shown on the left without the azido group) because the azido group destabilizes the negatively charged conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Carboxylic Acids | |
|
| 98 |
Go |
Q:
|
Given the two phenols below, which phenol is expected to have the lower pKa and why? The pKa refers to the alcohol site for deprotonation.
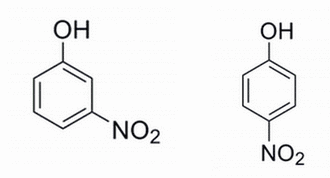 |
|
A
|
The molecule on the right will have the lower pKa and increased resonance stabilization
|
B
|
The molecule on the right will have the lower pKa and decreased resonance stabilization
|
C
|
The molecule on the left will have the lower pKa and increased resonance stabilization
|
D
|
The molecule on the left will have the lower pKa and decreased resonance stabilization
|
|
|
|
Tags:
Acid/Base Equilibria | Alcohols | |
|
| 99 |
Go |
Q:
|
Acetone is shown below on the left. What will happen to the pKa after the addition of an azido group to one of its methyl groups (actually replacing one of the methyl hydrogens)? The site of deprotonation (and thus where the pKa directly refers to) is the hydrogens on the carbon where the azido group was substituted (the -CH2). The modified molecule (after azido addition) is shown in the middle, and the azido group itself is shown on the far right.
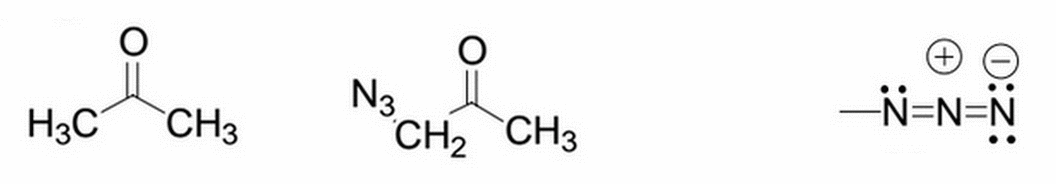 |
|
A
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be lower than the hydrogens on the molecule without the azido (the -CH3) because the azido group stabilizes the conjugate base
|
B
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be higher than the hydrogens on the molecule without the azido (the -CH3) because the azido group stabilizes the conjugate base
|
C
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be lower than the hydrogens on the molecule without the azido (the -CH3) because the azido group destabilizes the conjugate base
|
D
|
The pKa of the hydrogens of the azido-substituted molecule (the -CH2) will be higher than the hydrogens on the molecule without the azido (the -CH3) because the azido group destabilizes the conjugate base
|
|
|
|
Tags:
Acid/Base Equilibria | Aldehydes and Ketones | |
|
| 100 |
Go |
Q:
|
All individual amino acids are soluble in water; however, not all peptides are soluble. Which of the following choices best explains this? |
|
A
|
The R groups of the amino acid residues in the peptide are charged at physiological pHs
|
B
|
The R groups on all amino acids can interact noncovalently with water at pH = 7.4 (the pH of a normal, healthy body)
|
C
|
All peptides are insoluble in water
|
D
|
Individual amino acids are zwitterions at physiological pHs
|
|
|
|
Tags:
Protein Structure and Function | Acid/Base Equilibria | |
|
| 101 |
Go |
Q:
|
Which of the following statements is FALSE regarding primary and secondary amines? |
|
A
|
Primary amines are in general stronger bases than secondary amines. |
B
|
Primary amines are less sterically hindered than secondary amines. |
C
|
Primary and secondary amines both have a lone pair of electrons on the nitrogen atom. |
D
|
Primary and secondary amines can both be used in the production of an amide. |
|
|
|
Tags:
Molecular Structure | Acid/Base Equilibria | |
|
| 102 |
Go |
Q:
|
The nitrogen-containing reactant below (Reactant B) can act as a nucleophile in certain reactions. The reaction below is carried out in a pH = 4.5 buffer. This is because the pKa of the protonated carbonyl (Reactant A) is between -2 and -8, so there is a low concentration of this highly reactive electrophile at a pH of 4.5. If the pH is further lowered (from 4.5) in order to try and increase the concentration of this electrophile, which of the following correctly describes the likely counter-effect?
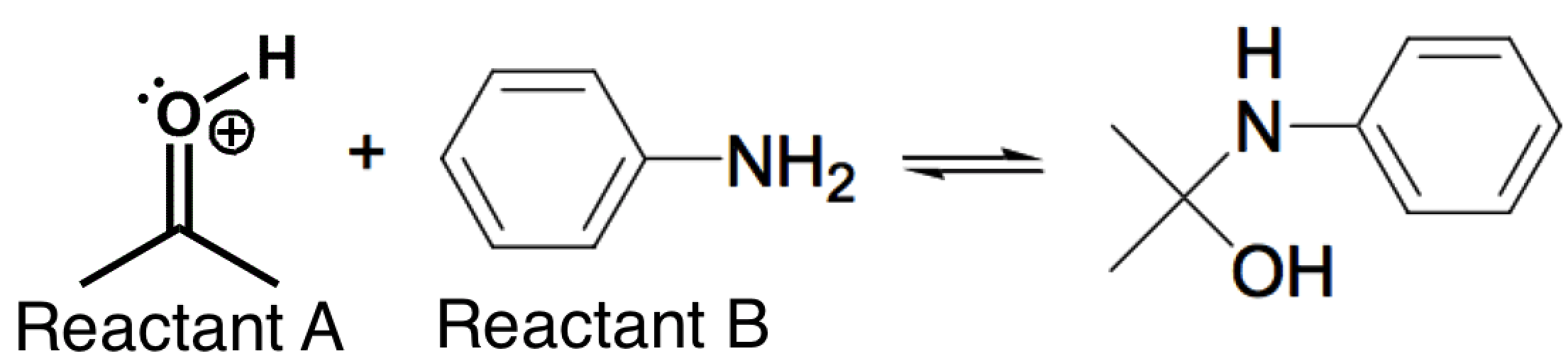 |
|
A
|
Increasing the acidity would cause less of Reactant B to become protonated (decreasing its nucleophilicity), and this would counteract the increase in electrophilicity of Reactant A.
|
B
|
Increasing the acidity would cause more of Reactant B to become protonated (decreasing its nucleophilicity), and this would counteract the increase in electrophilicity of Reactant A.
|
C
|
Increasing the acidity would cause more of Reactant B to become protonated and this would have no effect on the electrophilicity of Reactant A.
|
D
|
Increasing the acidity would have no effect on Reactant B and no effect on the electrophilicity of Reactant A.
|
|
|
|
Tags:
Acid/Base Equilibria | Miscellaneous Organic Chemistry | Organic Chemistry Reactions | Molecular Structure | |
|
| 103 |
Go |
Q:
|
A weak base is titrated with H2SO4. The equivalence point will occur at a pH that is: |
|
A
|
Less than 7 |
B
|
Exactly 7 |
C
|
Greater than 7 |
D
|
Dependent on the pKb of the base |
|
|
|
Tags:
Acid/Base Equilibria | Titrations | |
|
| 104 |
Go |
Q:
|
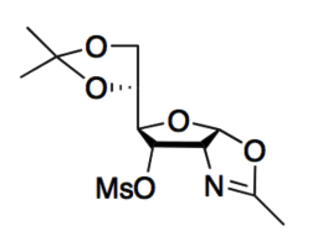
Given the molecule below, its most basic site:
 |
|
A
|
is the nitrogen because the conjugate acid can be stabilized by its resonance.
|
B
|
is the nitrogen because the conjugate acid is destabilized by its resonance.
|
C
|
is the oxygen (in the ring with the nitrogen) because the conjugate acid can be stabilized by its resonance.
|
D
|
is the oxygen (in the ring with the nitrogen) because the conjugate acid is destabilized by its resonance.
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 107 |
Go |
Q:
|
Which species of H3PO4 is least abundant at a pH of 2? |
|
A
|
H3PO4 |
B
|
H2PO4- |
C
|
HPO42- |
D
|
PO43- |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 108 |
Go |
Q:
|
Glutamic acid is only very slightly soluble in water. Its solubility can be best increased by: |
|
A
|
adding NaCl to the solution. |
B
|
decreasing the temperature of the solution. |
C
|
adding acid to the solution |
D
|
increasing the pH of the solution. |
|
|
|
Tags:
Solutions | Acid/Base Equilibria | |
|
| 109 |
Go |
Q:
|
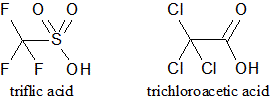
The structures of triflic acid and trichloroacetic acid are shown. How would the pKa of the two acids be compared? |
|
A
|
The pKa of triflic acid would be higher because it is a stronger acid. |
B
|
The pKa of triflic acid would be lower because it is a stronger acid. |
C
|
The pKa of trichloroacetic acid would be higher because it is a stronger acid. |
D
|
The pKa of trichloroacetic acid would be lower because it is a stronger acid. |
|
|
|
Tags:
Acid Derivatives | Carboxylic Acids | Acid/Base Equilibria | |
|
| 110 |
Go |
Q:
|
Which of the following is NOT a strong acid? |
|
A
|
H2SO4 |
B
|
HNO3 |
C
|
HClO4 |
D
|
HCOOH |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 111 |
Go |
Q:
|
Which of the following would happen to hexanoic acid in an aqueous solution when the pH his changed from 3 to 6? |
|
A
|
Hexanoic acid would polymerize. |
B
|
Hexanoic acid would become more protonated. |
C
|
Hexanoic acid would become more soluble. |
D
|
Hexanoic acid would precipitate. |
|
|
|
Tags:
Solutions | Carboxylic Acids | Acid/Base Equilibria | |
|
| 112 |
Go |
Q:
|
Which of the following is true when the pH of a solution is equal to the pKa of a monoprotic acid in the solution? |
|
A
|
The acid has been titrated to its equivalence point. |
B
|
50% of the acid has been deprotonated. |
C
|
The acid has been neutralized. |
D
|
The solution is neutral. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 113 |
Go |
Q:
|
What is the pOH of a solution composed of 0.001 M hydrobromic acid (a strong acid)? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 114 |
Go |
Q:
|
A particular weak acid molecule is found to have a pKa of 3.4. What is the ratio of this molecule's conjugated base to its acid form in the the blood, which has a pH of 7.4? |
|
A
|
0.003 |
B
|
4.77 |
C
|
400 |
D
|
10,000 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 115 |
Go |
Q:
|
Which of the following is the hydrogen ion concentration of a 3M solution of an acid with Ka of 3 x 10-6? |
|
A
|
5.5 x 10-4 |
B
|
4 x 10-4 |
C
|
6 x 10-3 |
D
|
3 x 10-3 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 116 |
Go |
Q:
|
An Bronsted-Lowry acid can be defined as: |
|
A
|
a substance which is capable of donating hydrogen ions. |
B
|
a substance which acts as an electron pair acceptor. |
C
|
a substance which acts as an electron pair donor. |
D
|
a substance which may act as both an acid as well as a base. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 117 |
Go |
Q:
|
A base is dissolved in solution such that the concentration of the base (X) is 100 mM and the concentration of the conjugate acid (HX+) is 1 M at equilibrium. If the pKb is 2.89, which of the following is closest to the pH of the solution? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 118 |
Go |
Q:
|
A titration takes place where a weak acid is added to a strong base. The pOH of the equivalence point of such a titration would be expected to be: |
|
A
|
less than 7. |
B
|
greater than 7. |
C
|
equal to 7. |
D
|
either equal to 7 or greater than 7. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 119 |
Go |
Q:
|
At room temperature, what is the quotient of the concentration of hydroxide ions to the concentration of hydrogen ions in a solution of pOH 3? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 120 |
Go |
Q:
|
Which of the following molecules is considered amphiprotic? |
|
A
|
hydrogen sulfate |
B
|
hydrochloric acid |
C
|
phosphate |
D
|
methane |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 121 |
Go |
Q:
|
Water autoionizes in an endothermic reaction with its Kw = 10-14 at 298 K. At 350 K, which of the following depicts the concentration of hydroxide ions? |
|
A
|
> 10-7 M |
B
|
10-7 M |
C
|
< 10-7 M |
D
|
The answer cannot be determined from the given information. |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 122 |
Go |
Q:
|
Experimental evidence has shown that increased s-character of a hybridized orbital results in a lower pKa of a C-H hydrogen. Which of the following hybridized orbitals would be expected to result in the most acidic C-H hydrogen? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 124 |
Go |
Q:
|
Which of the following is false regarding titration curves? |
|
A
|
when pH = pKa for an acid, there are equal protonated and deprotonated molecules |
B
|
diprotic acids can have one or two equivalence points |
C
|
as pH increases for an acid, more molecules become deprotonated |
D
|
the midpoint is the region most effective for buffering |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 125 |
Go |
Q:
|
Which of the following mixtures if added in a 1:1 ratio would result in the highest pOH? |
|
A
|
strong acid, strong base |
B
|
strong acid, weak base |
C
|
weak acid, strong base |
D
|
weak acid, weak base |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 126 |
Go |
Q:
|
At a temperature of 45 degrees Celsius, the equilibrium constant for water is 4-fold greater than that at 25 degrees Celsius. Which of the following is the concentration of hydrogen ions ([H+]) at 45 degrees Celsius? |
|
A
|
2*10-7 M |
B
|
2*10-14 M |
C
|
4*10-7 M |
D
|
4*10-14 M |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 127 |
Go |
Q:
|
Which of the following is the pH of an acid with pKa of 4.5 when the concentration of acid is equal to the concentration of the conjugate base? |
|
A
|
less than 4.5 |
B
|
4.5 |
C
|
greater than 4.5 but less than 7 |
D
|
greater than 7 |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 128 |
Go |
Q:
|
The Ka of a newly-developed acid is evaluated and noted to be unmeasurably high. What would be the expected pH of a 10M solution of this acid? |
|
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 129 |
Go |
Q:
|
A solution with pOH 10 is made from hydrochloric acid. What concentration of HCl was used to make this solution? |
|
A
|
0.0001 M |
B
|
0.001 M |
C
|
0.01 M |
D
|
0.1 M |
|
|
|
Tags:
Acid/Base Equilibria | |
|
| 130 |
Go |
Q:
|
What is the expected pH of a solution of sodium chloride? |
|
A
|
Greater than eleven |
B
|
Between seven and eleven |
C
|
Seven |
D
|
Less than seven |
|
|
|
Tags:
Acid/Base Equilibria | |
|
|
We can teach you how to crush the MCAT!
Learn More
|