|
| 1 |
Go |
Q:
|
What is the coefficient of NH3 when the equation NH3 + O2 → NO + H2O is balanced? Coefficients must be as small as possible. |
|
|
|
|
Tags:
Stoichiometry | |
|
| 2 |
Go |
Q:
|
In which of the following reactions will the equilibrium shift to the left when the pressure on the system is decreased?
I. 2H2 + O2 ↔ 2H2O
II. 2CO + O2 ↔ 2CO2
III. 2MgO(s) ↔ 2Mg(s) + O2(g) |
|
A
|
I only |
B
|
II only |
C
|
I and II only |
D
|
I and III only |
|
|
|
Tags:
Stoichiometry | Chemical Equilibrium | |
|
| 3 |
Go |
Q:
|
An overall reaction is made up of two elementary reactions. After the first step, the concentration of one particular species has increased. What could be the role of the species? |
|
A
|
Product |
B
|
Catalyst |
C
|
Intermediate |
D
|
Either A or C |
|
|
|
Tags:
Enzyme Structure and Function | Stoichiometry | |
|
| 4 |
Go |
Q:
|
The molecular mass of KOH is 56. In a reaction with another compound X, 1 mol of KOH is added to 1 mol of compound X. Compound X has a molecular mass of 200. The matrix of the reaction is only water, and upon rotary evaporation, 238 grams of product are recovered. Assuming 100% recovery of the product and a 100% completed reaction, what is the most likely explanation for the mass discrepancy? |
|
A
|
The caustic solution reacted with compound X in such a way that volatile compounds were liberated and later evaporated |
B
|
The K+ replaced the H on -OH, allowing the H to react with OH- to form water which was evaporated |
C
|
Two OH- for each KOH reacted with compound X to give a mass of 238 grams |
D
|
There is no mass discrepancy; 238 is the maximum amount of product that can be recovered from the reaction |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 5 |
Go |
Q:
|
What is the oxidation number of each Cl atom in Cl2O3. |
|
|
|
|
Tags:
Stoichiometry | |
|
| 6 |
Go |
Q:
|
In balancing the following equation, what is the value of x?
3 I2 + 6 KOH → KIO3 + x KI + 3H2O |
|
|
|
|
Tags:
Stoichiometry | |
|
| 7 |
Go |
Q:
|
What is the oxidation state of sulfur in HSO4-? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 8 |
Go |
Q:
|
Which of the following is a correctly balanced equation for the unbalanced reaction below?
Fe2O3 + CO → FeO + CO2 |
|
A
|
2Fe2O3 + CO → 2FeO + CO2 |
B
|
Fe2O3 + 2CO → 2FeO + CO2 |
C
|
Fe2O3 + CO → 2FeO + CO2 |
D
|
Fe2O3 + 3CO → 2FeO + 3CO2 |
|
|
|
Tags:
Stoichiometry | |
|
| 9 |
Go |
Q:
|
Elemental analysis of a compound shows its carbon content by mass is ~50%, its oxygen content by mass is ~33%, and its hydrogen content by mass is ~15% by mass. Which of the following empirical formulas is the closest to the empirical formula of the compound? |
|
A
|
C2H6O |
B
|
C2H4O |
C
|
C3H8O |
D
|
C3H6O |
|
|
|
Tags:
Periodic Table | Stoichiometry | |
|
| 10 |
Go |
Q:
|
In the following reaction, thionyl chloride (SOCl2) is a:
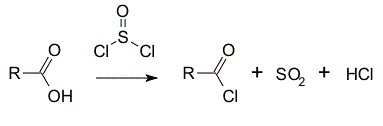 |
|
A
|
reactant |
B
|
product |
C
|
catalyst |
D
|
none of the above |
|
|
|
Tags:
Stoichiometry | |
|
| 11 |
Go |
Q:
|
What is the name of the structure below?
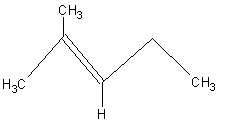 |
|
A
|
trans-2-pentene |
B
|
cis-2-pentene |
C
|
2-methyl-2-pentene |
D
|
trans-3-pentene |
|
|
|
Tags:
Stoichiometry | |
|
| 12 |
Go |
Q:
|
1 mole of C5H12 is combusted in a chamber containing 10 moles of O2. How many moles of water are produced, assuming complete combustion of the hydrocarbon? |
|
A
|
1 mole |
B
|
5 moles |
C
|
6 moles |
D
|
8 moles |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 13 |
Go |
Q:
|
A compound was analyzed and found to have 12.0 g carbon, 2.0 g hydrogen and 16.0 g oxygen. The molar mass of this compound is then determined to be 300.0 g. What is the molecular formula of the compound? |
|
A
|
C6H3O6
|
B
|
CH2O |
C
|
C12H6O12 |
D
|
C10H20O10 |
|
|
|
Tags:
Periodic Table | Stoichiometry | |
|
| 14 |
Go |
Q:
|
Which of the following is missing in the blank below?
Na2S2O5 + _______ → 2NaOH + 2SO2 |
|
A
|
H2O |
B
|
2H2O |
C
|
H3O+ |
D
|
OH- |
|
|
|
Tags:
Stoichiometry | |
|
| 15 |
Go |
Q:
|
What is the formal charge of the carbon in cyanide (CN-)? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 16 |
Go |
Q:
|
In the hydrolytic reaction between deuterated water (D2O) and a peptide, deuterium will be found on the products in which of the following bonds? |
|
A
|
-OH as -OD; -NH2 as -ND2 |
B
|
-OH as -OD; -NH2 as -NHD |
C
|
-OH as -OD only |
D
|
-NH2 as -NHD only |
|
|
|
Tags:
Stoichiometry | |
|
| 17 |
Go |
Q:
|
Magnesium and Copper (I) Sulfate are reacted to form Magnesium Sulfate and Copper. If 48 g of Magnesium are present initially and 32 g of Copper are formed, how many grams of Copper (I) Sulfate have reacted? |
|
A
|
40 g |
B
|
56 g |
C
|
115 g |
D
|
160 g |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 18 |
Go |
Q:
|
What is the percent yield of the reaction
3 Fe2O3 + CO → 2 Fe3O4 + CO2
if you start with 4 moles of Fe2O3 and 6 moles of CO, yielding 1.5 moles of Fe3O4? |
|
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 19 |
Go |
Q:
|
How many grams of CO2 are produced in the reaction of
Al2(CO3)3 → Al2O3 + CO2 (unbalanced)
if you start with 3 moles of Al2(CO3)3 and the reaction yield is 60%? |
|
A
|
5.4 g |
B
|
79 g |
C
|
238 g |
D
|
397 g |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 20 |
Go |
Q:
|
What is the net reaction of the three reactions shown?
1. 2NO + Cl2 → 2NOCl
2. N2O4 → 2NO2
3. 2NO2 → 2NO + O2 |
|
A
|
N2O4 + 2NO + 2NO2 + Cl2 → 2NOCl + 2NO2 + 2NO + O2 |
B
|
2NO + 2NO2 → 2NO2 + 2NO |
C
|
N2O4 + Cl2 → 2NOCl + O2 |
D
|
NO + Cl2 → 2NO + O2 |
|
|
|
Tags:
Stoichiometry | |
|
| 21 |
Go |
Q:
|
Zn + 2HCl → ZnCl2 + H2
What volume of gas is produced by the reaction above if 2 moles of HCl are used with excess Zn at STP? |
|
A
|
1 L |
B
|
2 L |
C
|
11.2 L |
D
|
22.4 L |
|
|
|
Tags:
Gases | Stoichiometry | |
|
| 22 |
Go |
Q:
|
Magnesium and copper (I) sulfate are reacted to form magnesium sulfate and copper. If 48g of magnesium are present initially and 32g of copper are formed, what is the percent yield of copper if copper (I) sulfate had been present in excess? |
|
A
|
5% |
B
|
12.5% |
C
|
45% |
D
|
62.5% |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 23 |
Go |
Q:
|
2 NaHSO3 + Zn → ________ + Zn(OH)2
In the balanced reaction above, which of the following is the correct compound in the missing blank? |
|
A
|
NaS2O4 |
B
|
Na2S2O4 |
C
|
Na2SO4 |
D
|
2 Na2SO2 |
|
|
|
Tags:
Stoichiometry | |
|
| 24 |
Go |
Q:
|
In the following set of reactions, if Al2(CO3)3 completely reacts and the aluminum oxide is completely reacted with HCl, what is the molar ratio of CO2 : H2O?
Al2(CO3)3 → Al2O3 + 3CO2
Al2O3 + 6 HCl → 2 AlCl3 + 3H2O |
|
|
|
|
Tags:
Stoichiometry | |
|
| 25 |
Go |
Q:
|
A 5 x 10-2 M solution of unknown identity at standard state at 298K is placed on one side of a semi-permeable membrane with pure water on the other side. Given that 3.6 atm of pressure is required to prevent the net flow of water across the membrane, what is the most likely identity of the unknown solution at standard state conditions? Osmotic Pressure ∏ = iMRT; where T is temperature, R = 0.08 L atm/mol·K, M is molarity, and i is the van't Hoff factor. |
|
A
|
Ca(NO3)2 |
B
|
NaCl |
C
|
ZnSO4 |
D
|
AlCl3 |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 26 |
Go |
Q:
|
Which of the following could be the molecular formula of a compound with the empirical formula CH2O? |
|
A
|
C8H12O8 |
B
|
C4H8O6 |
C
|
C3H6O3 |
D
|
C5H5O10 |
|
|
|
Tags:
Stoichiometry | |
|
| 27 |
Go |
Q:
|
Zn + HCl → ZnCl2 + H2
Consider the above unbalanced reaction. How many mL of a 4 M solution of HCl would be required to produce 22.4 L of H2 gas at STP?
|
|
A
|
125 mL |
B
|
250 mL |
C
|
500 mL |
D
|
750 mL |
|
|
|
Tags:
Gases | Stoichiometry | Quantitative Skills | |
|
| 28 |
Go |
Q:
|
What is the coefficient of O2 when the equation NH3 + O2 → NO + H2O is balanced? Coefficients must be as small as possible. |
|
|
|
|
Tags:
Stoichiometry | |
|
| 29 |
Go |
Q:
|
In HClO4, what is the oxidation number of the chlorine atom? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 30 |
Go |
Q:
|
In the combustion reaction below, how many grams of H2O are produced if 120 g of C4H10 are completely consumed?
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O |
|
A
|
190 g |
B
|
380 g |
C
|
600 g |
D
|
1200 g |
|
|
|
Tags:
Stoichiometry | |
|
| 31 |
Go |
Q:
|
Iron (relative atomic mass = 56) and sulfur (relative atomic mass = 32) can react in multiple ways to make various forms of iron (II) sulfur. Among the possible reactions is:
8Fe + S8 → 8FeS
Suppose 7 g of iron reacted with 4 g of sulfur in the reaction above. Which of the following statements would be true? |
|
A
|
Fe is the limiting reagent |
B
|
S8 is the limiting reagent |
C
|
FeS is the limiting reagent |
D
|
There is no limiting reagent |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 32 |
Go |
Q:
|
In the reaction below, hydrogen peroxide acts as the limiting reagent. If 680 grams of hydrogen peroxide are reacted, how many grams of water are produced?
IO3- + 2H2O2 + CH2(COOH)2 + H+ → ICH(COOH)2 + 2O2 + 3H2O |
|
A
|
360 g |
B
|
450 g |
C
|
540 g |
D
|
1020 g |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 33 |
Go |
Q:
|
The oxygen-evolving reaction Ca(OCl)2 (s) → CaCl2 (s) + O2 (g) is initiated with 71 g of Ca(OCl)2 in a 5.5 L sealed chamber with an unknown amount of inert gas. If the resulting oxygen evolved increases the pressure of the chamber by 20%, how many moles of gas were in the chamber initially? Assume all gases are ideal and the temperature of the reaction remains constant. |
|
A
|
0.5 moles |
B
|
2.5 moles |
C
|
3 moles |
D
|
11.2 moles |
|
|
|
Tags:
Stoichiometry | Quantitative Skills | |
|
| 34 |
Go |
Q:
|
In the reaction below, if 3 moles of HCl react completely with with excess NaHCO3, how much gas is evolved at STP?
HCl (aq) + NaHCO3 (aq) → NaCl (aq) + H2O (l) + CO2 (g) |
|
A
|
67.2 L |
B
|
7.5 L |
C
|
22.4 L |
D
|
11.2 L |
|
|
|
Tags:
Gases | Stoichiometry | Quantitative Skills | |
|
| 35 |
Go |
Q:
|
In the reaction NH3(g) + NO(g) → H2O(g) + N2(g), what is the coefficient in front of nitrogen gas in the stoichiometrically balanced equation? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 36 |
Go |
Q:
|
Which of the following is the empiric formula of an oxide compound where 75% of the weight of the molecule is arsenic? |
|
A
|
AsO |
B
|
AsO3 |
C
|
As3O2 |
D
|
As2O3 |
|
|
|
Tags:
Stoichiometry | |
|
| 37 |
Go |
Q:
|
The Haber Process is a system which converts hydrogen and nitrogen gas into ammonia. What is coefficient of hydrogen gas in the stoichiometrically balanced equation for the Haber Process? |
|
|
|
|
Tags:
Gases | Stoichiometry | |
|
| 38 |
Go |
Q:
|
Which of the following would NOT explain why the percent yield for a reaction could be calculated as less than 100%? |
|
A
|
A side reaction occurred. |
B
|
The reaction was incomplete. |
C
|
There was solvent contaminating the product. |
D
|
The reactants were impure. |
|
|
|
Tags:
Miscellaneous General Chemistry | Stoichiometry | |
|
| 39 |
Go |
Q:
|
In the balance equation for the combustion of propane (C3H8), which of the following is the correct coefficient for water? |
|
|
|
|
Tags:
Stoichiometry | Redox Reactions | |
|
| 40 |
Go |
Q:
|
Calcium chloride reacts in a double displacement reaction with silver nitrate. Which of the following is the coefficient in the balanced equation in front of silver chloride in the products? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 41 |
Go |
Q:
|
What is the mass percentage of carbon in nicotine (C10H14N2)? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 42 |
Go |
Q:
|
NH3 reacts with oxygen to form nitric oxide and water. If 51g of ammonia react with 64g of oxygen, how many grams of water would be expected to be formed? |
|
|
|
|
Tags:
Stoichiometry | |
|
| 43 |
Go |
Q:
|
How many sodium ions are present in 142 grams of sodium sulfate? |
|
A
|
6 x 1023 |
B
|
1024 |
C
|
1.2 x 1024 |
D
|
1.7 x 1024 |
|
|
|
Tags:
Stoichiometry | |
|
| 44 |
Go |
Q:
|
56 grams of C2H4 react with 16 grams of O2 to produce water and carbon dioxide. How many grams of water would be produced. |
|
|
|
|
Tags:
Stoichiometry | |
|
|
We can teach you how to crush the MCAT!
Learn More
|


