|
| 1 |
Go |
Q:
|
What is the standard cell potential of a cell composed of Li+ and F2, given the following equation and standard potentials?
F2(g) + 2Li(s) → 2F-(aq) + 2Li+
2Li+(aq) → 2Li(s) -3.04 V
F2(g) → 2F-(aq) 2.87 V |
|
A
|
-8.95 |
B
|
-5.91 |
C
|
5.91 |
D
|
8.95 |
|
|
|
Tags:
Electrochemistry | |
|
| 2 |
Go |
Q:
|
A battery consists of which type of cells? |
|
A
|
electrolytic |
B
|
electrochemical |
C
|
electroplating |
D
|
electromagnetic |
|
|
|
Tags:
Electrochemistry | |
|
| 3 |
Go |
Q:
|
An experimenter is working with an electrochemical cell, although he is unsure whether it is voltaic or electrolytic. There is a salt bridge for the cell composed of potassium iodide. What can the experimenter conclude? |
|
A
|
K+ will travel to the anode if the cell is electrolytic |
B
|
K+ will travel to the anode if the cell is voltaic |
C
|
I- will travel to the anode if the cell is electrolytic |
D
|
I- will travel to the cathode if the cell is voltaic |
|
|
|
Tags:
Electrochemistry | |
|
| 4 |
Go |
Q:
|
Which of the following can be used as a half-cell in an electrochemical cell along with Cu/Cu2+?
I. Al3+ + 3e- → Al (E0 = -0.76)
II. Cu2+ + 2e- → Cu (E0 = 0.34)
III. F2 + 2e- → 2F- (E0 = 2.87)
|
|
A
|
I and III only |
B
|
II only |
C
|
III only |
D
|
I, II and III |
|
|
|
Tags:
Electrochemistry | |
|
| 5 |
Go |
Q:
|
A galvanic cell is set-up between an Ag-AgCl electrode and standard hydrogen electrode. Some standard reduction potentials are provided. What is the potential across in this electrochemical cell?
2H+ + 2e- → H2 (E° = 0.00 V)
2H2O + 2e- → H2 + 2OH- (E° = -0.83 V)
Ag+ + e- → Ag (E° = 0.80 V)
AgCl + e- → Ag + Cl- (E° = 0.22 V)
|
|
A
|
0.44 V |
B
|
1.63 V |
C
|
0.22 V |
D
|
0.00 V |
|
|
|
Tags:
Electrochemistry | |
|
| 6 |
Go |
Q:
|
Given the following information:
Au3+ + 3e- → Au E° = 1.50 V
Cu 2+ + 2e- → Cu E° = 0.34 V
What is the standard potential of the following reaction? Is it spontaneous?
2Au + 3Cu2+ → 2Au3+ + 3Cu |
|
A
|
1.16 V, spontaneous |
B
|
-1.16 V, non-spontaneous |
C
|
1.98 V, spontaneous |
D
|
-1.98 V, non-spontaneous |
|
|
|
Tags:
Electrochemistry | Chemical Equilibrium | |
|
| 7 |
Go |
Q:
|
Given the cell half potentials below, what is the potential of a galvanic electrochemical cell in the reaction
Au3+ + Al(s) → Al3 + Au(s)
| Half Reaction | Potential (E°) |
| Au3+ + 3e- → Au(s) | +1.50 V |
| Al3+ + 3e- → Al(s) | -1.66 V |
|
|
A
|
-3.16 V |
B
|
-0.16 V |
C
|
0.16 V |
D
|
3.16 V |
|
|
|
Tags:
Electrochemistry | |
|
| 8 |
Go |
Q:
|
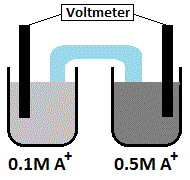
Which of the following is true regarding a concentration cell as the one depicted above, where both electrodes are composed of solid metal A? |
|
A
|
Electrons will flow from the half cell with the lower ion concentration to the half cell with the higher ion concentration. |
B
|
The salt bridge will transport A+ ions |
C
|
A+ ions will flow from the high-concentration electrode to the low-concentration electrode |
D
|
No voltage will be generated because the two half cells contain identical metals |
|
|
|
Tags:
Electrochemistry | Quantitative Skills | |
|
| 9 |
Go |
Q:
|
In a concentration electrochemical cell, two half-reactions contain the same electrode composition and ion content. The only difference is in the concentration of the ion solutions. This drives electron flow as the concentrations of the solutions reach equilibrium. In such a setup, which of the following is true? |
|
A
|
Electrons flow from the solution with higher concentration to the solution with the lower concentration |
B
|
Electrons flow from the cathode to the anode |
C
|
Electrons flow from the anode to the cathode |
D
|
Copper ions flow from the higher-concentration solution to the lower-concentration solution. |
|
|
|
Tags:
Electrochemistry | |
|
| 10 |
Go |
Q:
|
In an electrochemical concentration cell, two identical half reactions are set up with differing concentrations. This is different from galvanic cells which have two different half-cells in the circuit. In a concentration cell, the current is driven by which of the following? |
|
A
|
Electrostatic attraction of electrons to the cell of lower concentration.
|
B
|
Equalization of the cell concentrations due to entropy. |
C
|
Movement of metal ions from high to low concentration. |
D
|
Movement of metal ions from low to high concentration. |
|
|
|
Tags:
Electrochemistry | |
|
| 11 |
Go |
Q:
|
Using the standard half-cell potentials for the reactions below, identify the reaction which will be spontaneous.
Ba2+ + 2e- ↔ Ba -2.90 V
Mn2+ + 2e- ↔ Mn -1.04 V
K+ + e- ↔ K -2.92 V
2H+ + 2e- ↔ H2 0.00 V
I3- + 2e- ↔ 3I- 0.53 V |
|
A
|
Ba2+ + Mn ↔ Mn2+ + Ba |
B
|
K + Ba2+ ↔ K+ + Ba |
C
|
3I- + 2H+ ↔ I3- + H2 |
D
|
K+ + I3- ↔ 3I- + K |
|
|
|
Tags:
Electrochemistry | Chemical Equilibrium | |
|
| 12 |
Go |
Q:
|
If the following redox reaction is run in a galvanic cell, which species acts as the anode?
Li+ + Ag → Ag+ + Li (E° = -3.84 V) |
|
|
|
|
Tags:
Electrochemistry | |
|
| 13 |
Go |
Q:
|
| Half Reaction |
E° (V) |
| Fe3+ + e- →
Fe2+ |
+0.77 V |
| I2 + 2e- →
2I- |
+0.54 V |
| Mg2+ + 2e- →
Mg |
-2.36 V |
| Sn2+ + 2e- →
Sn |
-0.14 V |
With which metals listed above can iodine form a galvanic cell in which the iodine is oxidized? |
|
A
|
Iron only |
B
|
Magnesium only |
C
|
Magnesium and Tin only |
D
|
Iron and Tin only |
|
|
|
Tags:
Electrochemistry | |
|
| 14 |
Go |
Q:
|
Cu + 2Ag+ → Cu2+ + 2Ag
What is the standard potential of an electrochemical cell running the reaction above given the standard half-cell potentials below?
Cu2+ + 2e- → Cu +0.34 V
Ag+ + e- → Ag +0.80 V |
|
A
|
-0.46 V |
B
|
1.14 V |
C
|
0.46 V |
D
|
1.26 V |
|
|
|
Tags:
Electrochemistry | |
|
| 15 |
Go |
Q:
|
The process taking place at the anode of a cell is: |
|
A
|
reduction by a gain of electrons |
B
|
reduction by a loss of electrons |
C
|
oxidation by a gain of electrons |
D
|
oxidation by a loss of electrons |
|
|
|
Tags:
Electrochemistry | |
|
| 16 |
Go |
Q:
|
An anode can be described as: |
|
A
|
an electrode from which positive electric charges flow outwards. |
B
|
an electrode to which electrons flow inward. |
C
|
an electrode towards which positively charged ions move. |
D
|
an electrode towards which negatively charged ions move. |
|
|
|
Tags:
Electrochemistry | |
|
| 17 |
Go |
Q:
|
The cell potential for a voltaic cell (E) is +1.05V. Which of the following holds true for the Gibbs free energy for this reaction? |
|
A
|
It is positive |
B
|
It is negative |
C
|
It can be either positive or negative depending on the particular redox reaction |
D
|
It equates to 0 |
|
|
|
Tags:
Electrochemistry | Thermochemistry | |
|
| 18 |
Go |
Q:
|
The potential of a galvanic cell is measured at 0.36 V. If one of the half cell reactions is Cd2+ + 2e- → Cd (-0.40 V) which occurs as the reduction reaction. Which of the following could be the other half cell reaction? |
|
A
|
ClO3- + H2O + 2e- → ClO2- + 2OH- (0.36 V) |
B
|
Fe3+ + e- → Fe2+ (0.76 V) |
C
|
Fe2+ + 2e- → Fe (-0.36 V) |
D
|
Zn2+ + 2e- → Zn (-0.76 V) |
|
|
|
Tags:
Electrochemistry | |
|
| 19 |
Go |
Q:
|
Half reactions of several ions are calculated to determine the reduction potentials. For these ions, the reduction potentials are as follows: D>C>B>A. Which ion is the strongest oxidizing agent? |
|
|
|
|
Tags:
Electrochemistry | |
|
| 20 |
Go |
Q:
|
The Nernst equation: |
|
A
|
cannot typically be used to determine potentials of concentration cells. |
B
|
is independent of the number of electrons transferred in the cell reaction. |
C
|
relates electric potential to chemical concentrations. |
D
|
only provides electric potentials of galvanic cells at room temperature. |
|
|
|
Tags:
Electrochemistry | |
|
| 21 |
Go |
Q:
|
The reduction reaction for Ni2+ has a standard potential of -0.5 V and the reduction reaction for Al3+ has a standard potential of -1.5 V. If a galvanic cell is produced nickel at one terminal and aluminum at the other terminal, what is the absolute value of the standard cell potential for this cell? |
|
A
|
0.5 V |
B
|
1.0 V |
C
|
1.5 V |
D
|
2.0 V |
|
|
|
Tags:
Electrochemistry | |
|
| 22 |
Go |
Q:
|
The reduction potential for nickel, zinc, and aluminum are -0.23 V, -0.76 V, and respectively -1.66 V. Which of the following metals would be able to reduce aluminum when placed in an aqueous solution of aluminum cations? |
|
A
|
nickel only |
B
|
zinc only |
C
|
nickel and zinc |
D
|
neither nickel or zinc |
|
|
|
Tags:
Electrochemistry | |
|
| 23 |
Go |
Q:
|
Which of the following is true regarding galvanic and electrolytic cells? |
|
A
|
both types of cells drive spontaneous reactions |
B
|
concentration cells are a form of electrolytic cell |
C
|
both types of cells involve redox reactions |
D
|
in galvanic cells, oxidation occurs at the anode, however oxidation occurs at the cathode for electrolytic cells |
|
|
|
Tags:
Electrochemistry | |
|
| 24 |
Go |
Q:
|
Which of the following is true regarding concentration cells? |
|
A
|
the cells operate in absence of a salt bridge |
B
|
the Nernst equation can be used to measure the potential for these cells |
C
|
they are a subtype of electrolytic cells |
D
|
electrodes for each half-cell are composed of different compounds |
|
|
|
Tags:
Electrochemistry | |
|
| 25 |
Go |
Q:
|
Which of the following is the correct balanced chemical equation for the reaction that takes place at the anode of a lead storage battery? |
|
A
|
Pb2+ -> Pb |
B
|
Pb + SO42- -> PbSO4 + 2e- |
C
|
Pb -> Pb2 |
D
|
2e- + PbO2 + 4H+ + SO42- -> PbSO4 + 2H2O |
|
|
|
Tags:
Electrochemistry | |
|
|
We can teach you how to crush the MCAT!
Learn More
|

